9th Annual Retina ReportFollow the links below to read other articles from annual update on retina: The Bugs Behind Infectious Retinitis The Larry Alexander Resident Case Report Contest: Cloudy with a Chance of Retinopathy |
Retinal hemorrhages, often first diagnosed in the primary eye care setting, can be a presenting finding in many ocular and systemic disease states. Because of the blood-retinal barrier, which aids in isolating blood from the retina to avoid retinal toxicity, hemorrhages within the retina are rare unless there is insult to the ocular or systemic vasculature. Such damage can be caused by vascular disease, hematologic disorders and dyscrasias, infections, trauma or hypoxia. In addition, they can be idiopathic (Table 1).1 A look at the clinical presentation of retinal hemorrhages and the pathophysiology that leads to their correct identification and classification can help you work through the differential diagnoses and decide whether to monitor or refer.
 |
| Fig. 1. This preretinal hemorrhage forms a boat shape and blocks underlying retinal vasculature detail. |
Classification
Shape, location and size, as well as associated signs and symptoms, are all key to properly classifying a retinal hemorrhage. To begin, clinicians should document the location and depth with respect to the vitreal-retinal-choroidal interfaces. These, along with distribution of the hemorrhage, can give clues to the etiology and help uncover possible systemic comorbidities. Starting anteriorly, the classification of hemorrhages based on depth includes:
1. Vitreous and preretinal. A vitreous hemorrhage represents leakage of blood in and around the vitreous humor. Due to the lack of boundaries, these hemorrhages tend to diffuse throughout the vitreous and, in chronic cases, can settle inferiorly. Upon clinical evaluation, this may lead to a “hazy” appearing fundus due to the collection of red blood cells floating in the vitreous, especially in acute cases. A fresh vitreous hemorrhage often appears bright red, and turns to a more white or cream color due to red blood cell dehemoglobinization over time.
The subhyaloid is located between the posterior limiting layer of the vitreous and the internal limiting membrane (ILM) of the retina. Preretinal anatomical boundaries also exist between the internal limiting membrane and the retinal nerve fiber layer (RNFL). Thus, hemorrhages in both of these locations have more defined structural boundaries and are often indistinguishable (Figure 1). Like vitreous hemorrhages, subhyaloid and preretinal hemorrhages also block underlying retinal features and can reduce visual acuity when located near the macula.2
Preretinal, subhyaloid and vitreous hemorrhages are largely controlled by gravity. Preretinal and subhyaloid hemorrhages are darker at the bottom of the hemorrhage, where the blood has settled with a distinct horizontal line at the top, known as a “D” or boat shape.2
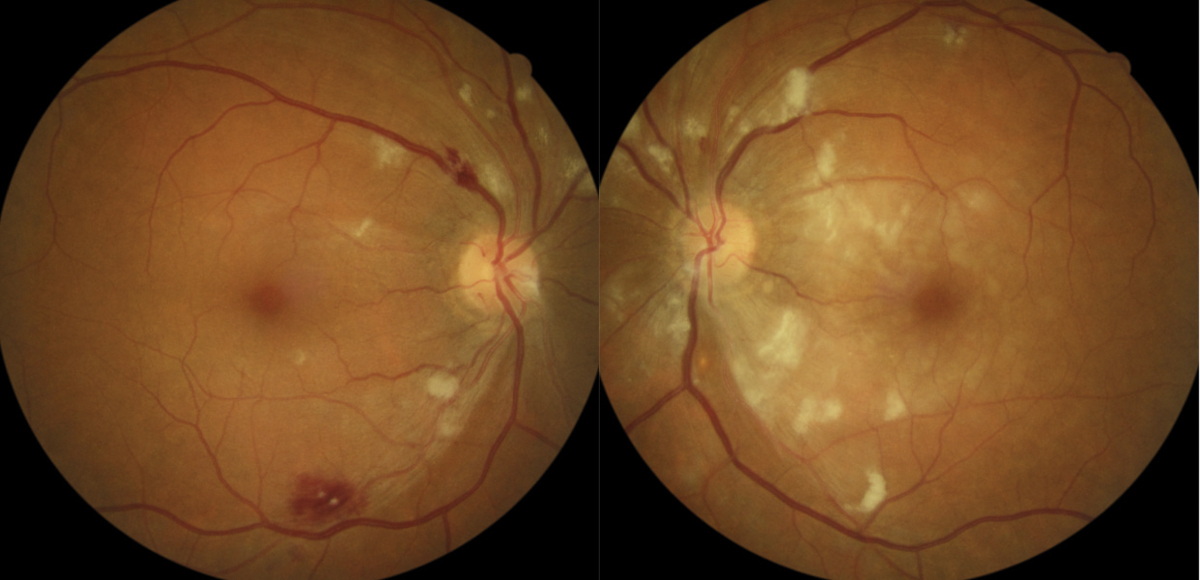
Due to their location, vitreous, subhyaloid and preretinal hemorrhages may be associated with abnormal retinal vessels, specifically in the superficial capillary plexus. These hemorrhages can also be associated with neovascularization of the nerve or retina as these leaky, fragile vessels often proliferate on the surface of the retina and scaffold onto the posterior hyaloid face. Other etiologies include traction from posterior vitreal separation, leading to a breaking or shearing of superficial retinal capillaries, as well as proliferative diabetic retinopathy, trauma, retinal break, vascular occlusion, Terson’s syndrome, hypertensive retinopathy and proliferative vitreoretinopathy. Certain cases may have no known etiology and are referred to as idiopathic.2,3
 |
| Fig. 2. RNFL hemorrhages can have an elongated shape, such as this one. |
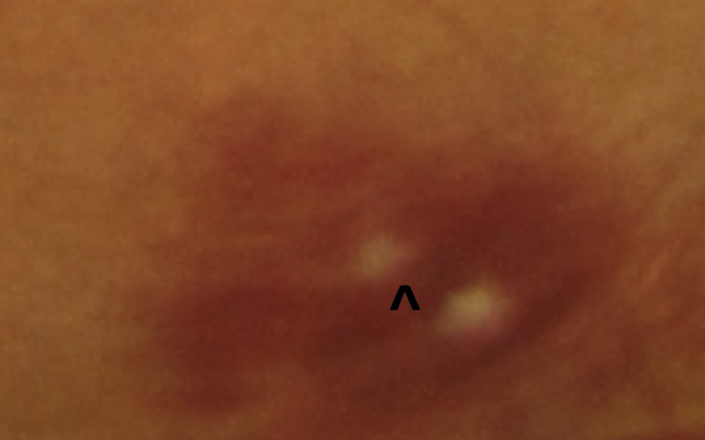 |
| Fig. 3. The white centers of this superficial RNFL hemorrhage are called Roth spots. |
2. RNFL. Hemorrhages in this location typically have a thin or elongated shape due to the parallel construction of ganglion cell axons and the distribution of RNFL bundles in the retina. These hemorrhages can be feather, flame, splinter or brush-stroke in appearance (Figure 2).2 They are often found in the posterior pole, last approximately six weeks and typically occur due to pathology of the superficial retinal capillary plexus.4,5 Researchers postulate flame-shaped hemorrhages are primarily affected by artery-based diseases such as hypertension, blood dyscrasias and anemias.2
Occasionally, a superficial hemorrhage may have a white center, in which case it is called a Roth spot, which can be caused by ischemia, fibrin accumulation or white blood cell accumulation (Figure 3).2,6 Roth spots are categorized as a non-specific sign of blood dyscrasia.7 Examples of associated etiologies include: anemia, anoxia, arteriovenous malformations, collagen vascular disease, diabetic retinopathy, hypertensive retinopathy, HIV, leukemia, multiple myeloma, trauma and radiation.
3. Intraretinal. Commonly known as “dot or blot,” these are found within the inner nuclear or outer plexiform layers of the retina. They fill the entirety of the retinal layers, occupying and displacing the normal retinal architecture, therefore forming round, uniform hemorrhages.2,7 The compressive force from the surrounding layers leads to the typical dot or blot shapes (Figure 4). Intraretinal hemorrhages appear slightly darker red than other hemorrhages. They typically have a pre-venular deeper capillary layer origin and therefore are associated with vein-based conditions or congestive disease. Conditions such as diabetes, venous occlusive disease, ocular ischemic syndrome, sickle cell retinopathy and juxtafoveal telangiectasia can lead to evidence of pathology in the deeper retinal layers.2
Table 1. Conditions that Can Present with Retinal Hemorrhages10,20-24 | ||
| Disease | Type of Retinopathy | Typical Laterality |
| Hypertension | Flame-shaped or intraretinal hemorrhages, microaneurysms/macroaneurysms, cotton wool spots, pre-retinal hemorrhage (rare). | Bilateral, can be asymmetric |
| Diabetes | Preretinal/vitreous or intraretinal (dot/blot) hemorrhages, microaneurysms, cotton wool spots. | Bilateral, can be asymmetric |
| Retinal Vein Occlusion | Flame-shaped or intraretinal hemorrhages, cotton wool spots, Bonnet’s sign. | Unilateral |
| Ocular/Head Trauma | Preretinal/vitreous, intraretinal or subretinal hemorrhages, choroidal neovascular membrane in cases of choroidal rupture. | Unilateral, depends on history/type of trauma |
| Anemia | Flame-shaped or intraretinal hemorrhages, cotton wool spots, Roth spots and vitreous hemorrhage in severe forms. | Bilateral |
| Leukemia | Preretinal/vitreous, flame-shaped or intraretinal hemorrhages, cotton wool spots, Roth spots, sea-fan neovascularization. | Bilateral |
| Acute Bacterial Endocarditis | Preretinal/vitreous, intraretinal or flame-shaped hemorrhages in the parapapillary rim, cotton wool spots, Roth spots. | Bilateral |
| Sickle Cell Retinopathy (SC & S-Thal, most common to have retinopathy) | Arteriole occlusions, intraretinal hemorrhages (pink or salmon patch), cotton wool spots, angioid streaks, black sunburst chorioretinal scars, sea-fan neovascularization with vitreous hemorrhage and tractional retinal detachment. | Bilateral |
| Connective Tissue Disorders (lupus) | Intraretinal hemorrhage, cotton wool spots, vascular occlusions (severe stages). | Bilateral |
| Pre-eclampsia | Intraretinal hemorrhages, cotton wool spots, Elschnig spots, arteriolar narrowing, serous detachment. | Bilateral |
| Ocular Ischemic Syndrome | Intraretinal hemorrhages (located mid-peripherally), cotton wool spots, arteriole narrowing, severe forms can develop retinal neovascularization. | Unilateral/usually asymmetric when bilateral |
| High Altitude Retinopathy | Vitreous and intraretinal hemorrhages, Roth spots, optic disc edema. | Bilateral |
| Carbon Monoxide Poisoning/Anoxia | Vitreous and intraretinal hemorrhages, Roth spots, optic disk edema. | Bilateral |
| Vitamin D Deficiency | Flame-shaped or intraretinal hemorrhage, cotton wool spots. | Bilateral |
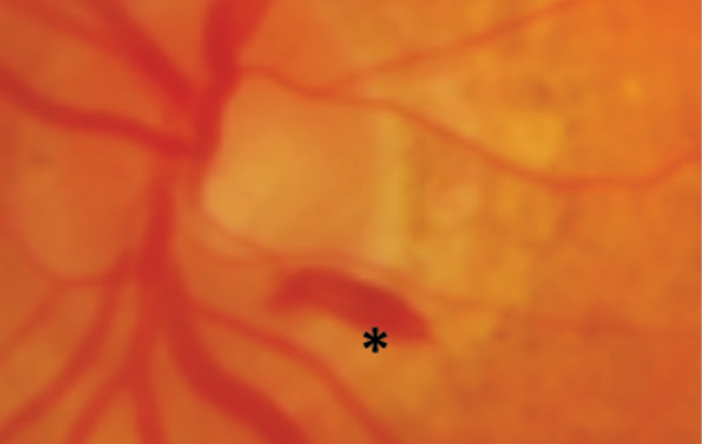
4. Subretinal. These hemorrhages, located underneath the photoreceptor layer of the retina and above the retinal pigment epithelium (RPE), are not confined laterally, which tends to make them appear broader with indistinct borders. They are confined anteriorly by the overlying retina and posteriorly by the tight junctions of the RPE. The hemorrhage is red, but not as bright a red as more superficial inner retinal hemorrhages due to their depth (Figure 5). Subretinal hemorrhages can be caused by choroidal neovascularization membranes (CNVM) types 1 through 4.
5. Sub-RPE. Located between the RPE and Bruch’s membrane, these appear dark red with defined borders (Figure 6). Based on the depth, the overlying retinal vessels can be visualized.2,8 A common etiology of these hemorrhages is a CNVM. However, clinicians must also rule out less common causes such as acute trauma, as in a choroidal rupture, or choroidal tumor.
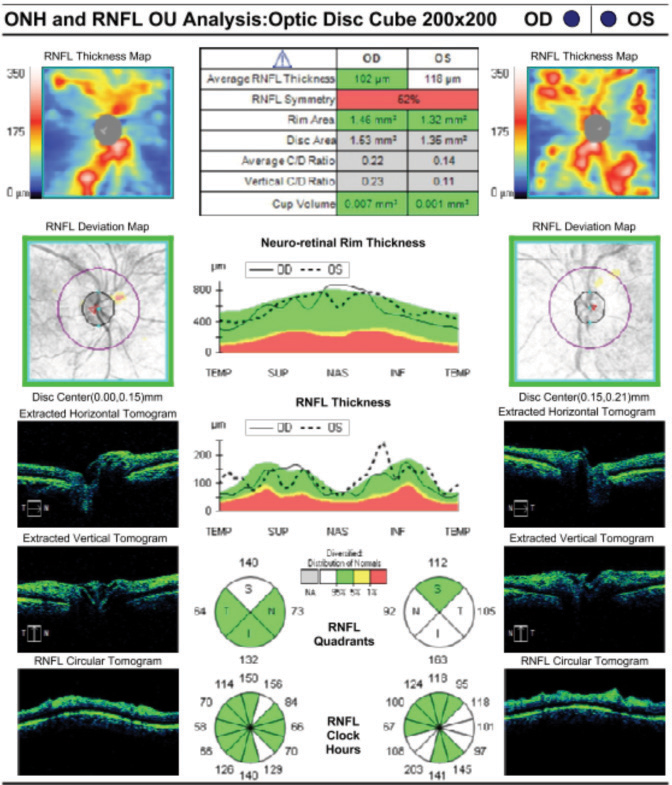
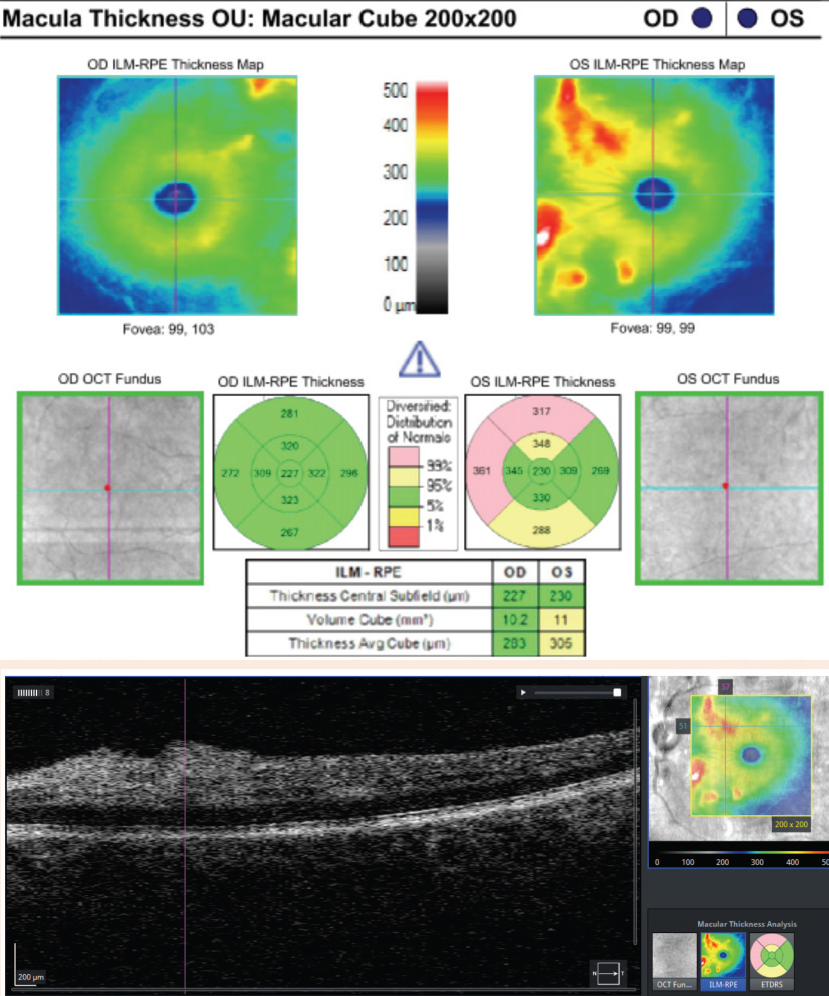
Case ExampleA 59-year-old African-American male presented with a history of vision loss OS, beginning two days earlier with gradual improvement. He reported a dull ache over his left eye but no other ocular symptoms. Systemically, he reported feeling extremely tired for the past few weeks with no other symptoms. He had a history of high blood pressure, arthritis and borderline hyperglycemia. He was taking atenolol 50mg daily for his blood pressure. He had an ocular history of an old blowout fracture OD, without any ocular sequelae. Visual acuities were 20/25 OD and 20/40-2 pinhole to 20/30 OS. Pupils were equal, round and reactive to light with no afferent pupillary defect OU. Intraocular pressures were 13mm Hg OD and 15mm Hg OS. Slit lamp exam was unremarkable. Dilated exam showed a cup-to-disc ratio of 0.2 vertical OD and 0.25 vertical OS with mild peripapillary edema OS. The macula was clear OD, but OS showed retinal thickening nasal to the fovea. Scattered RNFL and intraretinal hemorrhages and cotton wool spots (CWS) were noted in the posterior pole OS greater than OD. Presumed Roth spots were noted along the inferior temporal arcade OD (Figure 1). A peripapillary RNFL OCT confirmed peripapillary thickening OS greater than OD and a macular cube OCT confirmed mild inner retinal thickening nasal to the fovea OS (Figures 2 and 3). This thickening on OCT OU correlated to CWS OU in the RNFL of the retina. The patient was diagnosed with bilateral retinopathy of unknown etiology. Lab work ordered included complete blood count, BUN, HgA1c, sed rate, CRP, sickle cell screen, ACE, ANA, Lyme titer and RPR. The patient was scheduled in four days to review the lab work and order any additional testing if needed. Later that day, the lab notified the provider of critically low white blood cell count and low platelet and RBC counts. After coordinating with the on-call physician, the patient was transferred to the emergency room due to pancytopenia with concern for leukemia. A hematologist reviewed the case and recommended a bone marrow biopsy. The patient was discharged and a bone marrow biopsy was performed several days later, indicating acute myeloid leukemia. The patient was subsequently admitted for chemotherapy. |
Demographics
While trauma can cause retinal hemorrhages in any age group, bilateral hemorrhages in patients younger than three warrant suspicion of child abuse.1,9 Other differentials include: retinopathy of prematurity, persistent hyperplastic primary vitreous, Coat’s disease (acquired) and familial exudative vitreoretinopathy (inherited). Here, a thorough case history in regards to birth, trauma and any family history of ocular conditions is key.
Retinal hemorrhages in young adults are most commonly caused by Eales disease (male predilection), high altitude, Valsalva maneuvers or hematologic conditions caused by genetic disorders, anemias, blood cancers, sickle cell disease (higher frequency in people of African descent) or autoimmune conditions such as systemic lupus erythematous.10 In particular, almost half of patients with acute leukemia have retinal hemorrhages that are commonly intraretinal within the posterior pole and may be white-centered.11,12
Retinal vascular changes in young female patients may be associated with oral contraceptive use, as they can cause an increase in blood viscosity.13,14
Retinal hemorrhages in middle-aged and older patients are most commonly a result of systemic vascular conditions such as hypertension, diabetes and carotid occlusive disease, as well as vascular occlusions, posterior vitreous detachment and retinal tears.1,5,15
Patients older than 65 are at a greater risk of developing macular degeneration, and 30% of patients older than 75 have signs of maculopathy.16,17 This could result in the presence of retinal hemorrhages secondary to conversion to exudative macular degeneration. Also, peripheral exudative hemorrhagic chorioretinopathy, which may be a variant of age-related macular degeneration, has a mean onset of age 80 to 85.18 Patients within this age group are also at risk for retinal hemorrhaging secondary to manifestations of systemic conditions such as diabetes and hypertension, although one study shows hypertension explained less than half of retinal hemorrhages found in patients older than 60.19
 |
| Fig. 4. Intraretinal hemorrhages are round and uniform, often forming dot or blot shapes. Click image to enlarge. |
Table 2. Possible Laboratory Blood Workup for Retinal Hemorrhages25-31 | |
| Test | Indication |
| Complete blood count; Platelet count |
|
| Erythrocyte sedimentation rate; C-reactive protein |
|
| Fasting glucose; Hemoglobin A1c |
|
| Lipid profile |
|
| Prothrombin time/ partial thromboplastin time |
|
| Protein C and protein S |
|
| Factor V leiden |
|
| Prothrombin gene mutation |
|
| Homocysteine |
|
| Antithrombin III |
|
| Antiphospholipid antibodies |
|
| ELISA |
|
| Lyme titers; Herpes simplex titers; Syphilis testing FTA-ABS/RPR |
|
| ANA |
|
| Anti-dsDNA |
|
| Anti-RNP antibody testing |
|
| Anti-histone antibodies |
|
| Anti-Ro/SS-A, anti-La/SS-B antibodies |
|
| HLA-B5/B12 |
|
| HLA-B27 |
|
Diagnostics
Although a solitary retinal hemorrhage in one eye may not raise alarm, large interocular asymmetries in retinopathy should increase your suspicion and lead you to consider etiologies other than hypertension and diabetes.
One of the most crucial components of any ocular examination is a thorough case history, including social history. Medication use, history of trauma, history of recent Valsalva maneuvers, flashes or floaters and dimming/blacking out of vision are all pertinent when examining a patient with retinal hemorrhages. If no systemic history is available, begin differentiating based on the initial clinical assessment, which should include blood pressure, body mass index and blood sugar.
Optical coherence tomography, fluorescein angiography and fundus photography are all useful tools for determining the location and depth of the hemorrhage. Bloodwork also can be instrumental in determining the correct etiology, as well as any systemic associations (Table 2). Comanagement and care coordination with the patient’s primary care provider is essential, as is providing detailed education to ensure the patient is an active part of the team.
Ocular manifestations of systemic conditions can present asymmetrically, but most show some laterality. When an asymmetry in retinal hemorrhaging is noted absent ocular trauma, the carotid arteries should be evaluated via a carotid duplex ultrasound, computed tomography angiogram or magnetic resonance angiogram of the carotid arteries to rule out clinically significant stenosis or plaque formation. In some cases of carotid occlusive disease, chronic hypoperfusion to the retina on the ipsilateral side of the blockage of the internal carotid or ophthalmic arteries can lead to an asymmetry in retinopathy.
 |
| Fig. 5. The depth of this subretinal hemorrhage makes it less bright red than more superficial inner retinal hemorrhages. |
 |
| Fig. 6. Sub-RPE hemorrhages are often noted to be dark with well-defined borders, as seen here. |
Monitor or Treat?
Optometrists are tasked with determining whether the ocular findings are isolated or indicative of more widespread systemic disease. That decision will guide the clinician for proper treatment or even a potential referral for subspecialty care. ODs can follow these basic steps when triaging retinal hemorrhage patients:
1. Take a detailed medical and social history to determine potential correlations to the patient’s ocular findings. The patients’ known systemic comorbidities, current medications and activities of daily living may give the astute clinician valuable clues to an underlying etiology.
2. Determine whether the retinal hemorrhages are vision threatening based on the location to the fovea and other associated symptoms and ocular findings.
3. Generate a list of differential diagnoses. This will help to determine the need for referral to a retina specialist for further monitoring or treatment. The timing of the referral will depend on the potential for progression of the condition and any potential for visual loss. The differential diagnoses will also help comanagement with the primary care physician for continued coordination of ocular and systemic care with consideration for further testing, such as bloodwork.
4. Educate the patient about their ocular findings and any potential associations to systemic comorbidities. Patient education can be accomplished by means of drawings, software programs, reviewing of ancillary imaging results or other means that allow the patient to appreciate their eye findings in an easily interpreted manner. Convey to the patient the current risk the ocular findings have on their vision, as well as define the risks of progression and potential visual consequences.
5. Discuss a follow-up or referral time and schedule the patient to return to clinic with any new ocular symptoms or changes in vision.
Retinal hemorrhages are often first noted on comprehensive eye examinations during routine care. Often, these are correlated to known systemic or ocular comorbidities. In cases where no known associations can be elicited from the history or determined from clinical evaluation, further workup and comanagement with the primary care doctor may be necessary to determine the underlying etiology. A firm grasp of the classification of retinal hemorrhages, and knowing how to properly triage these patients, will ensure proper patient management.
Drs. DeMarco, Mah, Cole and Mazzarella are optometrists in North Carolina.
Contents of this publication do not represent the views of the US Department of Veterans Affairs or the United States Government.
1. Tolls DB. Peripheral retinal hemorrhages: a literature review and report on thirty-three patients. J Am Optom Assn. 1998;69(9):563-74. |