 Q: One of my patients has had multiple corneal graft failures and is now considering the Boston type 1 keratoprosthesis. What are some of the issues commonly encountered with this device, and do you have any tips on how to reduce such complications?
Q: One of my patients has had multiple corneal graft failures and is now considering the Boston type 1 keratoprosthesis. What are some of the issues commonly encountered with this device, and do you have any tips on how to reduce such complications?
A: The Boston type 1 keratoprosthesis, or KPro (Massachusetts Eye and Ear Infirmary), has undergone many upgrades in recent years, which have improved clinical outcomes and increased its use.1 “The main complications are difficulty in long-term management and glaucoma issues,” says Christopher J. Rapuano, MD, director of the Cornea Service Department and co-director of the Refractive Surgery Department at the Wills Eye Institute in Philadelphia.
Due to these risks, the patient will require rigorous, lifelong follow-up; however, keratoprostheses can produce good outcomes if the eye care provider stays vigilant and the patient remains compliant. “There are easily avoided minor complications in individuals who have had graft failures or who have normal ocular surfaces,” says James Aquavella, MD, professor of ophthalmology at the University of Rochester Flaum Eye Institute.
• Glaucoma. “Advancing glaucoma and blindness from glaucoma are major potential complications with a keratoprosthesis,” Dr. Rapuano says. “Most patients—either at time of KPro surgery or prior to it—have a tube shunt implanted, which decreases the chances of permanent glaucoma damage but doesn’t eliminate it.”
Because traditional tonometry cannot be used with the device in place, it is difficult to measure IOP accurately, and therefore, to follow the glaucoma in these patients. Dr. Aquavella suggests monitoring for IOP elevations using optic nerve head evaluation, visual fields and scleral indentation.
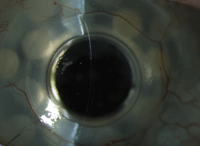
This patient has had a keratoprosthesis in place for five years.
• Ocular surface issues. Using a bandage contact lens improves device retention, ocular surface hydration and patient comfort and also prevents complications, such as delle formation, epithelial defects and corneal melt.1 “Contact lens management problems require refitting, frequent lubrication, and insertion and removal lessons,” Dr. Aquavella says. Some patients may need to use the bandage lenses indefinitely; if so, compliance is even more paramount.
• Endophthalmitis. The incidence of endophthalmitis in KPro eyes has decreased considerably with the use of long-term daily antibiotics, which typically include a fourth-generation fluoroquinolone and topical vancomycin.2 “Patients need to be very compliant with their contact lenses and with antibiotics permanently to decrease the risk of infection,” Dr. Rapuano says.
• Retroprosthetic membrane. The incidence of retroprosthetic membrane—membrane growth on the back side of the device—in KPro eyes is reported to be between 25% and 65%.3 “This is mitigated by topical steroids, with increasing dosage from the moment there are changes in the visual axis,” Dr. Aquavella says. “Ultimately, YAG laser treatment is easy to do, if necessary.”
• Cystoid macular edema. “Cystoid macular edema occurs in a small percentage and requires topical steroids,” Dr. Aquavella says. “The patient will require antibiotic prophylaxis forever along with follow-up every three months for the first year, and then every six months afterward.”
• Cellular debris. KPro patients are also prone to accumulating cellular debris within and around the PMMA back plate. “Small amounts of debris can severely reduce vision,” Dr. Aquavella says. “Debris on the optic can be cleared with routine cleansing or the use of gas permeable lens cleaner.”
1. Magalhães FP, Sousa LB, Oliveira LA. Boston type I keratoprosthesis: Review. Arq Bras Oftalmol. 2012 May-Jun;75(3):218-22.
2. Nouri M, Terada H, Alfonso EC, et al. Endophthalmitis after keratoprosthesis: incidence, bacterial causes, and risk factors. Arch Ophthalmol. 2001;119(4):484-9.
3. Dohlman CH, Colby KA, Belin MW, Todani A. Titanium vs. PMMA backplates for Boston keratoprosthesis: incidence of retroprosthetic membrane. ARVO, 2009;1505/A415.

