Annual Cornea ReportFollow the links below to read other articles from our annual Cornea Report: Fixing a Hole: How to Heal Persistent Epithelial Defects |
To preserve patients’ vision and ocular health, primary eye care providers need to stand confidently alongside ophthalmologists to assist in postoperative management. With respect to corneal pathology, restoring vision can now be achieved, in some cases, by applying corneal transplants using less risky, more predictable procedures.
As transplant procedures become safer and more precise, more are performed both in the United States and worldwide.1 According to the Eye Bank Association of America, 79,304 keratoplasties were performed in 2015, an increase of 3.75% from 2014.1
As optometrists managing corneal disease, our job is to be well-versed in cornea transplant options, educate the patient, make the appropriate referrals and actively participate in patients’ postoperative care.
The term corneal transplant is no longer synonymous with full-thickness penetrating keratoplasty (PKP). It is now divided into subcategories with different tissue layers. This family of procedures includes traditional PKP, deep anterior lamellar keratoplasty (DALK), Descemet’s stripping endothelial keratoplasty (DSEK/DSAEK), Descemet’s membrane endothelial keratoplasty (DMEK) and pre-Descemet’s endothelial keratoplasty (PDEK).
This article reviews these options, what patients need to know and the optometrist’s role in comanagement.
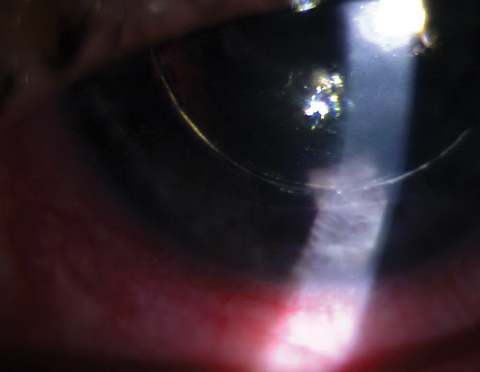 |
| DSEK with gas/air bubble posterior to the iris causing pupillary block. Click image to enlarge. |
Penetrating Keratoplasty
A traditional PKP involves the removal of all cornea layers, which essentially leaves the globe open for a period of time (called an “open sky” procedure) until the donor tissue can be secured. Next, the donor graft is attached using four interrupted cardinal sutures, secured sequentially 180 degrees from one another, ensuring proper tension on the graft to minimize induced astigmatism. Next, either more interrupted sutures are placed in the primary clock hours or, in some cases, a long, single running suture is placed in a circumferential fashion to assist in securing the donor tissue to the host. Some surgeons prefer placement of a combination of interrupted and running sutures.
The advent of femtosecond laser technology has made a significant impact on the world of anterior segment surgery—from the creation of LASIK flaps to employment in cataract surgery for precutting the capsulorhexis and “prechopping” the lens prior to phacoemulsification. During corneal transplants, a femtosecond laser may also be used in place of the corneal trephine to create the initial incisions into the host cornea, as well as cutting of the donor button. The graft-host junction may be manipulated and customized to a specific architecture, theoretically providing a more secure interface with greater surface area.
In a procedure as invasive as a full-thickness PKP, suprachoroidal hemorrhage is an intraoperative risk. The incidence of this complication ranges from 0.1% to 1.08%.2 If the eye is not closed quickly, total prolapse of the contents of the eye may occur, resulting in complete vision loss.2 Postoperative PKP risks include retinal detachment, endophthalmitis, glaucoma, cataract, ocular surface disease, infectious keratitis, graft dehiscence, graft failure and graft rejection.3
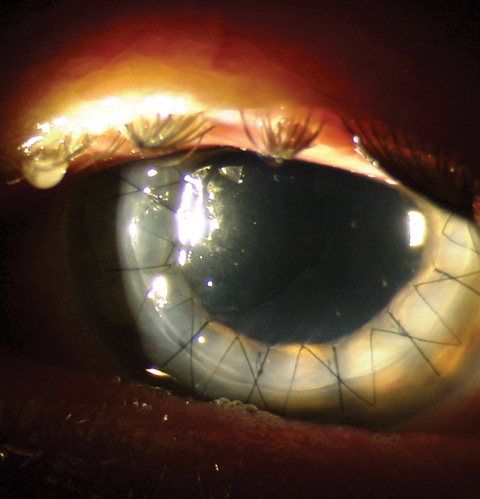 |
| This postoperative patient demonstrates full-thickness penetrating keratoplasty graft with a running suture in place. Click image to enlarge. |
PKP Postoperative Care
Following the procedure, medications include topical immunosuppression, antibiotic coverage and lubrication in support of the ocular surface. Common corticosteroids following PKP include prednisolone acetate 1% and Durezol (difluprednate, Novartis). Typically, these medications are maintained for several months to years following surgery to modulate the recipient’s immune response to graft tissue. These topical drugs may be supplemented by systemic immunomodulatory agents such as CellCept (mycophenolate mofetil, Genentech) in patients at high risk for graft rejection.4
A typical graft appearance on day one will show moderate graft edema throughout the donor tissue and the margins of the host rim. In the first few weeks following the procedure, control, tissue integrity maintenance and ocular surface recovery are the focus. Patients may also have a wide variety of pain from persistent foreign body sensation to more intense aching, soreness and photophobia. Generally, these symptoms improve over the first few days. Persistent or increasing inflammation over the first few days, especially in association with anterior chamber reaction or presence of hypopyon, may indicate infection.
As the new cornea stabilizes over the first two to four weeks, vision will gradually improve. You may encounter issues such as high or irregular astigmatism. In eyes that do not show improvement of edema and vision, monitor for signs of primary graft failure.5
Endothelial rejection is a leading cause of graft failure.5 Symptoms of this will include redness, photophobia, pain and blurred vision.5 The clinical signs of a graft rejection include decreased acuity, conjunctival hyperemia, corneal edema, subepithelial infiltrates and keratic precipitates, often in a pathognomonic pattern called a Khodadoust line.5
Risk of graft rejection is increased with corneal neovascularization, and researchers have tested drugs to help minimize the vascular ingrowth. One study shows complete regression of deep stromal vessels in 16 patients using Avastin (bevacizumab, Genentech), partial regression in six and improved visual acuity in five.6 Researchers have looked into in various delivery methods for Avastin, including topical, subconjunctival and intrastromal injections.6
 |
| As opposed to the pristine DMEK patient (at right), you can see this detached DMEK is rolled up in the patient’s anterior chamber. Click image to enlarge. |
Lamellar Keratoplasties
The goal of all posterior lamellar keratoplasties is to remove the diseased endothelial pump cells while leaving unaffected layers intact. Healthy endothelial cells are critical for stromal deturgescence and corneal clarity. These transplants offer improved quality of vision and ocular health to patients with endothelial diseases such as Fuchs’ dystrophy and pseudophakic bullous keratopathy.7 These transplants also offer the advantage of keeping more native anatomy in place, decreasing the antigenic burden of foreign donor tissue.
In DSEK, the surgeon removes the host Descemet’s membrane and endothelium before inserting a donor graft of posterior stroma, Descemet’s and endothelium. The incision—through which the graft is inserted—is larger and is closed by the surgeon with one dissolvable suture. Finally, an air or sulfur hexafluoride (SF6) gas bubble is inserted into the anterior chamber and the patient is positioned with their nose pointed to the ceiling, capitalizing on gravity to tamponade the graft into place. Topical steroids are used to maintain graft clarity and prevent rejection episodes. Steroids dosed four times per day in the early postoperative period is common, then tapering down over one year.
Postoperative complications can be segmented into two stages: early (one to 28 days postoperatively) and late (28 days and later). In the early stage, adverse events include problems with graft adhesion and iatrogenic pupillary block secondary to the anterior chamber. We typically instruct patients to maintain a supine position as often as possible after the procedure to maximize the tamponade effect of the air bubble on the graft position.
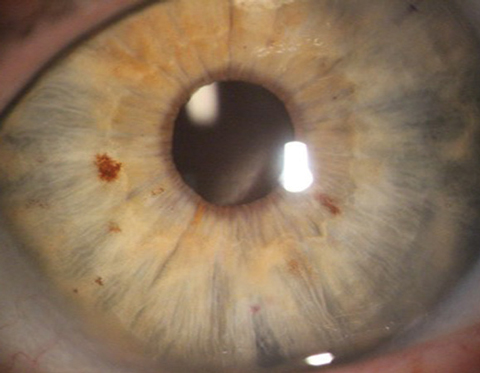 |
| This is how a pristine post-DMEK patient should appear. |
An inferior peripheral iridotomy (PI) is placed in endothelial keratoplasties (EK), but if the bubble is blocking the PI, the iridotomy is non-patent or the bubble moves posterior to the iris causing chamber shallowing, iatrogenic pupillary block with a sky-high intraocular pressure (IOP), headache and nausea/vomiting can ensue.
Symptoms of pupillary block are similar to acute angle-closure glaucoma and include headache, nausea, blurred vision and halos.8 You can initiate a simple intervention when these patients first call the office by having the patient sit up, a position that will cause the air bubble to rise. If in 20 minutes to 30 minutes the patient experiences no relief, they must be seen. During an office visit, a corneal surgeon will manipulate the bubble with a partial removal through the corneal wound or paracentesis (similar to “burping” a wound). Once the bubble diffuses out of the anterior chamber (approximately four to seven days after the procedure) EK grafts should attach to the host tissue. In instances when they don’t, the surgeon may opt to place a second air bubble at the slit lamp with subsequent patient positioning. In rare cases, the surgeon may need to refloat the graft in the operating room or consider a graft exchange if graft attachment is unlikely through in-office manipulations. Once the problem has been resolved, the patient needs to be monitored carefully until the gas bubble has dissolved and they are no longer at risk.
In all these procedures, it is critical to monitor IOP at each visit because the patient will be on steroid therapy for an extended period. If IOP increases in the short term, use a topical IOP agent such as Combigan (brimonidine/timolol, Allergan) or Simbrinza (brinzolamide/brimonidine, Novartis) to decrease ciliary body production while the steroid is tapered. Cosopt (dorzolamide/timolol, Akorn) is also an option.
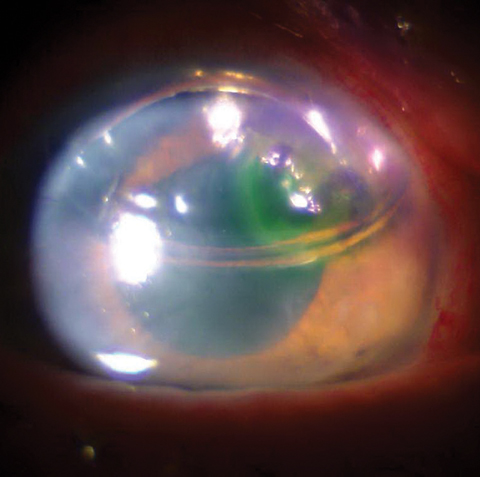 |
| This photo shows a one-day postoperative DSEK patient with bubble in place. |
Dealing With Rejection
Late-stage complications and management involve restoring visual acuity and preventing graft rejection or failure. In comparison with PKP, DSEK offers fewer corneal sutures and more native anterior corneal tissue, resulting in less total astigmatism.9 Generally, the thinner the transplant tissue and the more host anatomy left in place results in better acuity and less risk of graft rejection.9,10 A review of DSEK found astigmatic shift post-DSEK to be near neutral at 0.11D.9 Clinically, less induced astigmatism coupled with a thinner graft offers faster recovery and better potential visual acuity in DSEK patients when compared with PKP.9-11
If a rejection episode does occur, treat aggressively with strong topical steroids such as Durezol (difluprednate, Novartis) or Pred Forte (prednisolone acetate, Allergan) dosed up to every hour as first-line treatment.
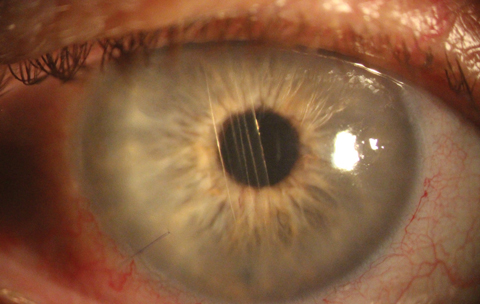
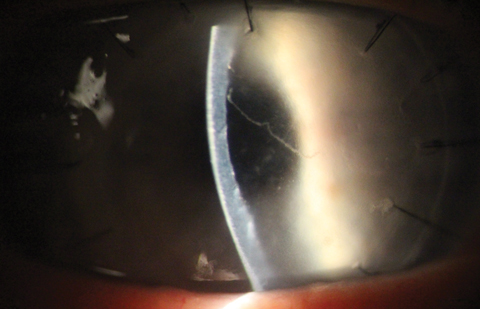 |
| Khodadoust line and corneal graft rejection as seen in a PKP patient. Click image to enlarge. |
Comparing Techniques
Research suggests DSAEK does better than PKP when it comes to risk for rejection.12 A five-year survival study of DSAEK vs. PKP in a large cohort of Asian eyes with Fuchs’ dystrophy and bullous keratopathy shows statistically significant differences in graft survival, endothelial cell loss, graft rejection and wound dehiscence.12 Another study shows that outcomes were stronger for patients who had a failed PKP and underwent a second full-thickness keratoplasty than for patients whose doctors replaced only the diseased posterior layers of the failed transplant.13
DMEK is an even thinner transplant for patients with endothelial disease. In DMEK, the surgeon removes the patients Descemet’s membrane and endothelium and inserts a donor button of Descemet’s membrane and endothelium. Similar to DSEK, one dissolvable suture is placed and an air or sulfur hexafluoride gas bubble is used for graft tamponade. Again, in DMEK a postoperative steroid is used to maintain transplant health, tapering over one year without stopping. In our clinic, the steroid is tapered over one year before stopping with the same guidelines for graft rejection episodes occurs.
The visual acuity achieved with DMEK is generally superior to DSAEK.7,11,14 This may be due in part to DMEK’s use of a thinner graft with more native corneal anatomy, less induced hyperopia and less induced higher-order aberrations.7,11,14
A disadvantage of DMEK, however, is graft dislocations. The literature documents higher graft re-bubble rates compared with DSEK.7 Researchers suspect better graft adhesion in DSEK is due to additional stromal tissue from the graft.7
Lastly, PDEK involves Dua’s layer. This graft is similar to DMEK with an additional 10µm to 15µm anterior to Descemet’s membrane. This new approach is not widely performed or studied thus far. but the hope is this variation provides the benefits of both DMEK and DSEK.15
Corneal transplant surgery has undergone several changes throughout the years and continues to evolve alongside innovations in technology. The movement towards lamellar grafts and replacing only the diseased tissue instead of the entire cornea has been beneficial for graft survival, as well as recovery times and improved visual outcomes for patients. As primary eye care providers with increasing patient demands, it is imperative that we comanage corneal transplantation when necessary and can make appropriate referrals for our patients to achieve optimal outcomes.
Dr. Ibach specializes in advanced anterior segment surgery care and pathology at Vance Thompson Vision in Sioux Falls, SD. He is a fellow of the American Academy of Optometry and a member of the American Optometric Association. He has a consulting agreement with Alcon.
Dr. Hauswirth is an assistant professor at the University of Colorado School of Medicine. He is an active industry consultant and speaker. Relevant disclosures: Alcon, Allergan, Bausch+Lomb, BioTissue, Shire, Sun and TearScience.
1. Eye Bank Association of America. 2015 Eye Banking Statistical Report. Washington, DC; Eye Bank Association of America;2015. |

