 |
New research published in Diabetes Care evaluating the effects of blood glucose levels on the progression of diabetic retinopathy (DR) has shown that a nearly four-year period (3.7 years) of tight glycemic control cut the risk of retinopathy by nearly 50% eight years from the time of study enrollment—an example of a “legacy effect,” also known to researchers as “metabolic memory.”1
The study investigated whether rigid glycemic control, combined with fenofibrate for dyslipidemia and intensive blood pressure control, reduced retinopathy progression—seen in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye Study—four years after the trial was completed.1
Evaluating for retinopathy progression using the Early Treatment Diabetic Retinopathy Study (ETDRS) scale, only 5.8% of the subjects receiving tight glycemic control—defined as A1c level at less than 6.0%—progressed three or greater steps. Those subjects who received only the standard level of glycemic control—A1c between 7.0 to 7.9% —saw significantly greater progression—12.7%.1
Intensive blood-pressure control—120mm Hg vs. a control of 140mm Hg—showed no effect on retinopathy in either the ACCORD or ACCORDION studies.
Emily Y. Chew, MD, deputy director of the National Eye Institute's (NEI) division of epidemiology and clinical applications presented the findings on June 11 at the American Diabetes Association 2016 Scientific Sessions. “This study sends a powerful message to people with type 2 diabetes who worry about losing vision,” said Dr. Chew in an NEI press release.2 “Well-controlled glycemia, or blood sugar level, has a positive, measurable, and lasting effect on eye health,” Dr. Chew noted.
The Fenofibrate Study Arm
When the ACCORD study evaluated 3.7 years of fenofibrate therapy (160mg/day) in conjunction with simvastatin, the risk of retinopathy progression was reduced compared to treatment with simvastatin alone. However, the ACCORDION follow-up study showed no significant difference between the two cohorts (11.8% in the fenofibrate plus simvastatin group vs. 10.2% in simvastatin-only group).1This finding suggests that fenofibrate treatment may need to be ongoing to maintain benefit, but “further evaluation is required,” Dr. Chew said in the NEI statement.
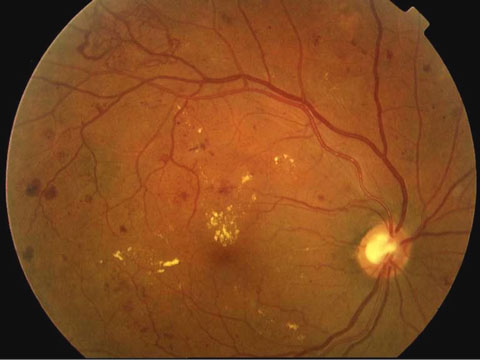 |
| The ACCORD Eye Study analyzed 2,856 ACCORD participants who had not received laser photocoagulation or vitrectomy for proliferative diabetic retinopathy. |
Diabetes expert Paul Chous, OD, of Tacoma, Wash. says that the risks in this study arm certainly differ from the glycemic control arm. “While fenofibrate didn’t show a legacy effect against retinopathy, the drug significantly lowered risk for patients during the course of the study. These findings align, he says, with those of the FIELD study in Australia where fenofibrate is a “first-line therapy against retinopathy progression with a relatively low number needed to treat—14—to prevent one person from needing photocoagulation or anti-VEGF injections.”
Clinical Takeaway
“ACCORDION once again shows that a relatively short period of tighter blood glucose control—a mere 45 months—as reflected by A1c, provides protection against the development or progression of diabetic retinopathy in the future, despite worsening A1c over time,” says Dr. Chous.
He notes that ACCORD and ACCORDION parallel earlier studies in both type 1 and type 2 diabetes mellitus. “The DCCT/EDIC studies in type 1 diabetes and the UKPDS in type 2 diabetes showed the same beneficial ‘legacy effect’ after about eight to 10 years of tighter blood glucose control.” (Editor’s note: The aforementioned studies are the Diabetes Control and Complications Trial, the Epidemiology of Diabetes Interventions and Complications, and the United Kingdom Prospective Diabetes Study.)
Hopefully, the legacy effect shown in ACCORDION persists longitudinally in the same vein as its predecessors. “What is unique about ACCORDION is the finding that a legacy effect applies in patients with high cardiovascular risk, and for the briefest time span studied to date (a 3.5 year treatment duration and four-year follow-up),” Dr. Chous says. The benefit was sustained over time in the previous studies—up to 30 years in DCCT/EDIC—so it will be interesting to see if the beneficial effects found in ACCORDION will persist past the four-year mark, he remarks.
“The quagmire faced by PCPs, endocrinologists, cardiologists and eye doctors alike,” Dr. Chous says, is the original mortality finding by ACCORD—tighter glucose control in patients with high cardiovascular risk increases the risk for death. “ACCORD saw 22% increased mortality in the tight glucose control arm—though the larger European ADVANCE trial and the Veterans Association Diabetes Trial did not in similar high-risk populations,” says Dr. Chous. Of note, the elevated risk of death in the high-risk cohort was partly due to acute hypoglycemia. “This correlated with longer diabetes duration,” Dr. Chous remarks.
Dr. Chous says that on the whole, optometrists need to strive to prioritize patients’ systemic health within the context of diabetes.
“We need to encourage newly diagnosed diabetes patients to get tighter control of their blood sugar as soon and for as long as is safely possible—hopefully before they develop heart disease.” For patients at high cardiovascular risk, he says, “we must balance the severity of baseline retinal findings against the risk of death.”
He says to individualize blood sugar targets in accordance with age and duration of the condition. “An 80-year-old patient with type 2 diabetes for 30 years and no retinopathy but with history of myocardial infarction will not likely benefit, and may very likely be harmed, by an A1c under 6.5%.” By taking into account the insights gleaned from this new study and applying them to each patient, optometrists can minimize risk of retinopathy and help to preserve overall good health.

