 |
One of the true emergencies in all of eye care is a patient suffering vision loss from giant cell arteritis (GCA). Vision loss comes in the form of ischemic optic neuropathy or retinal artery occlusion. In many cases, what begins as unilateral devastating vision reduction quickly progresses to bilaterality and total visual disability for the patient.1,2 Treatment is high-dose systemic steroids, either oral or inpatient intravenous infusion.
Prevalence
Patients suffering from GCA have a mean age of 71 years at presentation.3 The prevalence increases with increasing age.3 This condition is generally considered only after the age of 50. Women are somewhat more likely to develop GCA, and it is much more common in Caucasians.4
A multitude of systemic manifestations can signal the presence of GCA, including malaise, weight loss and anorexia, headache (typically in the temporal or occipital region), pulseless and indurated temporal arteries, night sweats, tongue necrosis and oral ulceration, dental abscess, scalp pain and scalp necrosis, jaw claudication when eating, head and neck swelling, anemia, depression, mental disturbance, neck pain, low-grade fever, transient ischemic attack and stroke, proximal myalgia, breast masses, gynecological disorders, malignant disease, persistent flu-like illness, chronic pharyngitis, vertigo, muscle aches, cardiac arrhythmia, congestive heart failure and myocardial infarction.5-13
Pathophysiology
GCA is a granulomatous inflammation of medium- and large-sized arteries that have a defined internal and external elastic lamina.5 The interleukin-6 (IL-6) pathway is up-regulated in GCA. There is cellular infiltration of the muscular wall of these vessels by T-lymphocytes, macrophages, histiocytes, plasma cells and multinucleate giant cells.14,15 The resultant inflammation fragments the vascular walls and leads to collapse of the vessel lumen with resultant ischemia.
Unquestionably, systemic steroids are needed to preserve vision and reduce morbidity and mortality.16-18 One report recommends that patients with vision loss or other ocular complications receive three to four daily infusions of 250mg of methylprednisolone for three days.19 For oral administration, the initial prednisolone dose is 60mg/d to 80mg/d. It should be reduced in weekly steps of 5mg to 10mg until 20mg/d, and by 2.5mg until 10mg/d.16-18 Dose reduction is 1mg/month below 10mg/d, depending on symptoms and erythrocyte sedimentation rate or C-reactive protein. Suppression of the disease usually takes months to years, leaving patients and physicians to cope with complications of long-term steroid use such as ulcers and gastrointestinal bleeding, osteoporosis, increased risk of heart disease, diabetes, decrease in bone density, increased risk of infections, thin skin, easier bruising and slower wound healing. A steroid-sparing therapy would help patients and physicians greatly.
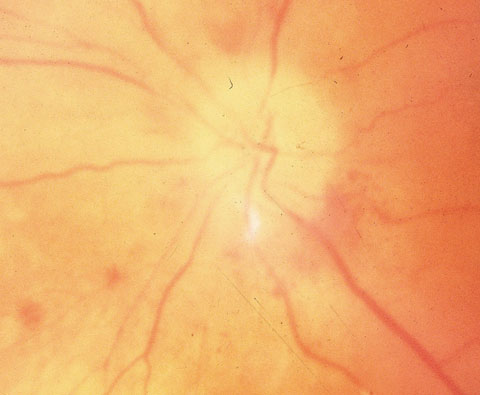 |
| A pale, swollen disc with parapapillary hemorrhages in arteritic anterior ischemic optic neuropathy. |
The Search For New Therapies
One of the greatest difficulties in finding new therapies for GCA involves the direct consequences of inadequately treating the disease in clinical trials. It is well known that steroids are an effective treatment for GCA; hence, it would be medically unethical for a study to directly compare steroids to any medication whose efficacy is unknown or merely theoretical due to the devastating consequences that subjects may experience.16-19 Thus, all study treatments have to be performed in conjunction with steroids to see if a combination with steroids would be superior to steroids alone. This is the only way the efficacy and role of a newly tested medication can be assessed in a safe and appropriate fashion.
Recently, the FDA granted a breakthrough therapy designation to Actemra (tocilizumab, Genetech) for the treatment of GCA. This is the first innovative therapy for GCA in more than 50 years. The breakthrough therapy designation is designed to speed the development for treatments of serious diseases such as GCA and certain cancers.
The FDA designation was based upon unpublished results of the GiACTA trial, a multicenter, randomized, double-blind, placebo-controlled study designed to test the ability of tocilizumab, an IL-6 receptor antagonist, to maintain disease remission in patients with GCA.20 GiACTA data will be submitted for presentation at an upcoming medical conference and to the FDA for approval consideration.
Patients were randomized to receive tocilizumab 162mg weekly injections plus a six-month and 12-month prednisone-taper compared with controls receiving placebo plus similar steroid taper. The preliminary results indicate that patients receiving high dose tocilizumab had superior disease remission at one year compared with the steroid-only taper. Further investigation from this study will attempt to identify the lowest therapeutic dose of prednisone that can be used in patients also using tocilizumab, the amount of tocilizumab needed to induce remission and how long patients stay in remission on this therapy.
GCA is a devastating disease that afflicts approximately 200,000 Americans annually. Steroid treatment, while effective, has its own negative impact on patients. We now stand on the cusp of a new medication which promises to at least reduce the amount of steroids used in treatment with an expected reduction in side effects. Perhaps tocilizumab will be proven through studies to not only be an adjunct to steroids, but even perhaps a replacement.
|
1. Hayreh SS, Podhajsky PA, Zimmerman B. Ocular manifestations of giant cell arteritis. Am J Ophthalmol. 1998;125(4):509-20. 2. Wein FB, Miller NR. Unilateral central retinal artery occlusion followed by contralateral anterior ischemic optic neuropathy in giant cell arteritis. Retina. 2000;20(3):301-3.Nordberg C, Johansson H, Petursdottir V, et al. The epidemiology of biopsy-proven giant cell arteritis: special reference to changes in the age of the population. Rheumatology 2003; 42: 549-52. 3. Salvarani C, Gabriel SE, O’Falon WM, et al. The incidence of giant cell arteritis in Olmstead County, Minnesota: apparent fluctuations in a cyclic pattern. Ann Intern Med 1995; 123:192-4. 4. Rahman W, Rahman FZ. Giant cell (temporal) arteritis: an overview and update. Surv Ophthalmol. 2005;50(5):415-28. 5. Zachariades N, Skoura C, Spanou A, et al. Temporal arteritis: report of a case. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102(2):192-7. 6. Gurwood AS, Brilliant R, Malloy KA. The enigma of giant cell arteritis: multidisciplinary management of two cases. J Am Optom Assoc. 1998;69(8):501-9. 7. Liozon E, Ouattara B, Portal MF, et al. Head-and-neck swelling: an under-recognized feature of giant cell arteritis. A report of 37 patients. Clin Exp Rheumatol. 2006;24(2 Suppl 41):S20-5. 8. Gonzalez-Gay MA, Garcia-Porrua C, Llorca J, et al. Visual manifestations of giant cell arteritis. Trends and clinical spectrum in 161 patients. Medicine (Baltimore). 2000;79(5):283-92. 9. Nordborg E, Nordborg C. Giant cell arteritis: strategies in diagnosis and treatment. Curr Opin Rheumatol. 2004;16(1):25-30. 10. Liozon E, Loustaud V, Fauchais AL, et al. Concurrent temporal (giant cell) arteritis and malignancy: report of 20 patients with review of the literature. J Rheumatol. 2006;33(8):1606-14. 11. Manetas S, Moutzouris DA, Falagas ME. Scalp necrosis: a rare complication of temporal arteritis. Clin Rheumatol. 2006 May 6; [Epub ahead of print]. 12. Biebl MO, Hugl B, Posch L, et al. Subtotal tongue necrosis in delayed diagnosed giant-cell arteritis: a case report. Am J Otolaryngol. 2004;25(6):438-41. 13. Albert DM, Searl SS, Craft JL. Histologic and ultrastructural characteristics of temporal arteritis. The value of the temporal artery biopsy. Ophthalmology 1982; 89:1111-26. 14. Ashton-Key M, Gallagher PJ. Surgical pathology of cranial arteritis and polymyalgia rheumatica. Baillieres Clin Rheumatol 1991;5:387-404. 15. Scheurer RA, Harrison AR, Lee MS. Treatment of Vision Loss in Giant Cell Arteritis. Curr Treat Options Neurol. 2011 Oct 27. [Epub ahead of print]. 16. Gonzalez-Gay MA, Martinez-Dubois C, Agudo M, et al. Giant cell arteritis: epidemiology, diagnosis, and management. Curr Rheumatol Rep. 2010;12(6):436-42. 17. Schmidt J, Warrington KJ. Polymyalgia rheumatica and giant cell arteritis in older patients: diagnosis and pharmacological management. Drugs Aging. 2011;28(8):651-66. 18. Schmidt WA. Current diagnosis and treatment of temporal arteritis. Curr Treat Options Cardiovasc Med. 2006;8(2):145-51. 19. Unizony SH, Dasgupta B, Fisheleva E, et al. Design of the tocilizumab in giant cell arteritis trial. Int J Rheumatol. 2013;2013:912562. |

