 A 48-year-old white male presented as a new patient in January 2012 with a simple complaint of blurred vision with near tasks. He wore spectacles with a compound hyperopic, astigmatic and presbyopic correction that was two years old.
A 48-year-old white male presented as a new patient in January 2012 with a simple complaint of blurred vision with near tasks. He wore spectacles with a compound hyperopic, astigmatic and presbyopic correction that was two years old.
Systemic medical history was remarkable for hypertension, hypercholesterolemia and hyperlipidemia. He has a significant family history of cardiovascular disease, and he’d had a coronary artery bypass graft about two years earlier. Current medications included Prinivil (lisinopril, Merck), Lipitor (atorvastatin, Pfizer), Cardizem (diltiazem, Valeant) and Plavix (clopidogrel, Bristol-Myers Squibb). He reported no allergies to medications.
He said that at his last eye examination, the doctor mentioned that he had glaucoma in his right eye and that he should be “taking drops” to treat it. He reported that he did begin a drop (he couldn’t remember which) once daily at bedtime in the right eye, but discontinued it after a week or so due to irritation. He admitted that he should have followed earlier recommendations, and that he was now prepared to do so.
Diagnostic Data
Entering visual acuity was 20/25- OD and 20/25+ OS at distance and J3 OU at near; he was correctable to 20/20 OU. Pupils were equal, round and reactive to light with no afferent defect. Extraocular motilities were full in all positions of gaze.
Slit lamp examination of his anterior segments was unremarkable in both eyes, and his angles were estimated as grade 4 open OU. Central corneal thicknesses measured 531µm OD and 526µm OS. Intraocular pressure was 29mm Hg OD and 22mm Hg OS.
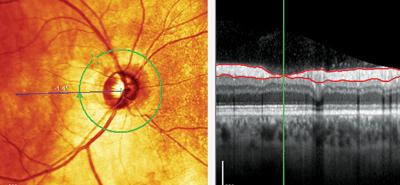
Note the retinal nerve fiber layer (RNFL) wedge defect seen at 10 o’clock (left). The RNFL thickness scan (right) shows the green line at the center of the wedge defect, where the RNFL was only 4μm thick.
Upon dilation, his optic nerves showed glaucomatous optic neuropathy OD. Specifically, the cup-to-disc ratio of the right eye was 0.55 x 0.85 with marked thinning of the superior neuroretinal rim, where there was a notable nerve fiber layer wedge defect. Cup-to-disc ratio of the left eye was 0.45 x 0.50 with a plush, well-perfused neuroretinal rim.
The retinal vasculature showed moderate hypertensive and arteriosclerotic (AS) retinopathy (OD slightly worse than OS). Specifically, the retinal arterial tree in the right eye was characterized by thinned retinal arterioles along the superior arcade, with grade 1 hypertensive retinopathy and grade 3 AS retinopathy. The vasculature of the left eye was comparable, with an overall decreased arteriole-to-venule (A/V) ratio, grade 1 hypertensive retinopathy and grade 2 AS retinopathy. The peripheral retinal examination revealed scattered areas of microcystoid OU.
At the initial visit, stereo optic nerve images were obtained as baseline. We scheduled the patient for optic nerve imaging and visual fields in two weeks, and prescribed new glasses.
At the follow-up, the patient’s IOP measured 31mm Hg OD and 23mm Hg OS. Visual fields demonstrated paracentral areas of decreased sensitivity in the inferior arcuate area OD, consistent with the neuroretinal rim thinning. Gonioscopy revealed grade 4 open angles OU with minimal trabecular pigmentation.
Management
I prescribed Travatan Z (travoprost, Alcon) OD HS, and asked him to follow up in three to four weeks. On this and further follow-up visits, the patient was compliant with his appointments and reported good compliance with his drop regimen. His average IOP during this time was 18mm Hg to 20mm Hg OD. I had set a target IOP of 16mm Hg or less in the right eye, so I ultimately added timolol 0.5% OD QD a.m.
Subsequent visits have shown that the IOP, neuroretinal rim, RNFL and visual fields in his right eye have stabilized. The left eye is also stable with no medical intervention.
Discussion
This patient presented with three notable findings: glaucomatous optic neuropathy OD, notable asymmetry between the optic nerves, and a significant history of cardiovascular disease.
We know that patients with vasculospastic disease and poor ocular perfusion are at an increased risk of developing glaucoma. This patient’s history of significant cardiovascular disease correlates with the possibility of poor perfusion to the eye. And the patient had undergone carotid Doppler imaging, which was normal right and left.
While various formulas exist to estimate ocular perfusion pressure, they all take into account blood pressure as measured in the arm. But, this does not always correlate to blood pressure and perfusion at the level of the central retinal artery—especially in the presence of carotid artery disease.
Also, we know that pressure in the optic nerve subarachnoid space plays a role in perfusion pressure-related mechanics of the optic nerve head—a pressure that’s difficult at best to measure, and realistically can only be estimated via imaging. (
See “Can High Pressure Be Helpful?” December 2012).
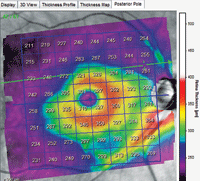
Significant thinning in the superior temporal arcuate area extends from the optic nerve beyond the temporal side of the foveal avascular zone.
So, in the absence of definitive carotid artery disease, are there any other findings—at least from a vascular perspective—that suggest an increased risk of developing glaucoma?
The answer now seems to be yes. For many years, we’ve known that decreased retinal arterial diameter is often associated with glaucoma, but the data was confounded by other risk factors.1-3 But recently, a 10-year study—part of the Blue Mountains Eye Study—examined the relationship between retinal vessel diameter (both arterioles and venules) and the risk of developing glaucoma.4
After accounting for the correlation between IOP, elevated cholesterol and ocular perfusion pressure, the authors found a significant relationship between decreased retinal vessel caliber diameter and the risk of developing glaucoma.4
Importantly, this study showed a significant relationship between the risk of developing glaucoma and decreased retinal artery diameter after eliminating confounding factors. No relationship was found between decreased retinal vein diameter and glaucoma.
So, does the presence of decreased retinal arterial diameter play a role in determining the risk of developing glaucoma? Absolutely. But, further work is needed to specifically outline the significance of decreased retinal artery diameter. For the question still remains: Does the decreased retinal arterial diameter and, presumably, the concurrent decreased perfusion, cause the glaucomatous optic nerve damage that we see—or does the optic nerve damage of the neuroretinal rim and the retinal nerve fiber layer ultimately result in less need for perfusion?
In any case, we must be vigilant for signs of decreased optic nerve and perioptic nerve perfusion, such as decreased arteriolar diameter. And, because retinal arterial thinning can cause loss of tissue at an area located “away” from the neuroretinal rim, maybe we need to look more closely there for early damage—especially because the technology to do so now exists.
1. Zheng Y, Cheung N, Aung T, et al. Relationship of retinal vascular caliber with retinal nerve fiber layer thickness: the Singapore Malay Eye Study. Invest Ophthalmol Vis Sci. 2009 Sep;50(9):4091-6.
2. Mitchell P, Leung H, Wang JJ, et al. Retinal vessel diameter and open-angle glaucoma: the Blue Mountains Eye Study. Ophthalmology. 2005 Feb;112(2):245-50.
3. Ikram MK, de Voogd S, Wolfs RC, et al. Retinal vessel diameters and incident open-angle glaucoma and optic disc changes: the Rotterdam study. Invest Ophthalmol Vis Sci. 2005 Apr;46(4):1182-7.
4. Kawasaki R, Wang JJ, Rochtchina E, et al. Retinal vessel caliber is associated with the 10-year incidence of glaucoma: the Blue Mountains Eye Study. Ophthalmology. 2013 Jan;120(1):84-90.

