Innovations in Eye CareCheck out the other feature articles in this month's issue:Bioengineering the Retinal Pigment Epithelium Iontophoresis: Wave of the Future Can We Lower IOP with Glasses? While You Were Sleeping |
Upon initial diagnosis, a new glaucoma patient typically faces some grim realities. They learn that their neuro-ophthalmic infrastructure is already damaged—and irreversibly so—because as much as 40% of retinal ganglion cell (RGC) degeneration occurs before the condition is even clinically distinguishable through visual field changes.1,2 They are also highly vulnerable to further vision loss. And not a single treatment in the clinician’s arsenal can undo any of the damage.
Population-wide, the prospects are no better. Glaucoma is responsible for 15% of all blindness with over 500,000 new cases each year.3,4 In the United States alone, 2.9 million people are affected, representing 2.1% of the population over the age of 40.5 These estimates are only predicted to worsen as the population ages.
It is common to detect retinal neurodegeneration only after extensive RGC death and significant visual loss have already occurred. Given the primacy of RGCs in glaucoma’s story, our research team has been working on a promising new diagnostic approach to improve early diagnosis that detects glaucomatous changes at a cellular level.
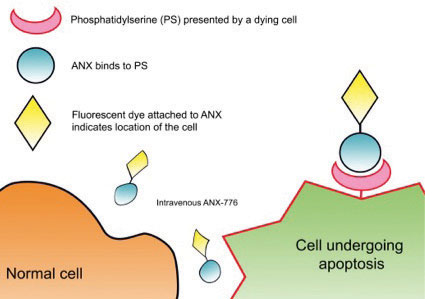 |
| Fig. 1. This diagram shows where annexin-5 binds to an apoptosing cell compared with a normal cell. |
Detecting Cell Death
Recent advances in OCT technology have given clinicians the ability to measure retinal nerve fiber layer (RNFL) and ganglion cell complex (GCC) thickness, but of course the process begins earlier than that, at the cellular level. What we see in RNFL or GCC dropout is axonal loss. But, what of the cells themselves? Our approach images the process of apoptosis, or “programmed cell death” as it occurs. In so doing, we hope to provide the eye care community with tools to identify clinically modifiable targets before RNFL loss happens, possibly even allowing us to stave off that previously unavoidable consequence.
Apoptosis occurs in all multicellular organisms, permitting cell death without necrosis and subsequent inflammation. However, pathogenic altered rates of this process are implicated in hematologic, cardiovascular, neurodegenerative (Alzheimer’s, Parkinson’s) and retinal (AMD) diseases.6-14 In early apoptosis, the cell membrane changes its structure, and phosphatidylserine, a membrane phospholipid, moves from within the cell to the outer surface. The protein annexin-A5 has been extensively used in cell biology to assess apoptosis in cancer, stroke and heart disease.15 It contributes to the regulation of membrane permeability and repair, making it an ideal marker of cellular predisposition to apoptosis.
We have developed a novel technique called DARC (detection of apoptosing retinal cells) to monitor this process of retinal cell death in vivo. Using a fluorescently labeled variant of annexin-A5, a protein that binds to the exposed phosphatidylserine, and because the eye is transparent, we were able to visualize the fluorescence and identify individual apoptosing cells (Figure 1). The eye is unique in this ability due to the transparent nature of the optical media.
This led to the DARC project, a unique collaboration between Imperial College London and University College London, funded through the Wellcome Trust. In the Phase I clinical trial, safety and tolerability of DARC was assessed; however, we also wanted to compare whether there was a different signal between healthy controls and those who had progressing glaucoma. Sixteen subjects—eight healthy volunteers and eight with early, progressing glaucoma—were included in the trial.
Annexin-776 (ANX776), especially created for this application, was intravenously administered similar to the procedure used for a fluorescein or indocyanine green angiogram.15 Before and following injection with ANX776, real-time images using near-infrared confocal scanning laser ophthalmoscopy captured retinal images at zero, 15, 30, 60, 120, 240 and 360 minutes. To establish dose safety and efficacy, we administered ascending doses of 0.1mg, 0.2mg, 0.4mg and 0.5mg in four single-dose groups of patients. Retinal imaging and pharmacokinetic studies were performed over six hours. The resultant images displayed hyperfluorescent spots appearing on the retina, thought to represent individual apoptosing retinal cells.
As far as we are aware, this milestone was the first experiment of its kind to visualize in vivo retinal apoptosis in humans. Importantly, ANX776 was found to be safe and well-tolerated with a short half-life of 30 minutes.
A Phase II trial has now been performed in patients with glaucoma, AMD, optic neuritis (multiple sclerosis), Down’s syndrome (who display similar pathological features as in Alzheimer’s disease) and healthy volunteers to further characterize the differences in spot count, distribution and morphology between diseases (Figure 2). These four diseases all lack techniques to achieve early diagnosis and monitor disease activity, a barrier to developing effective neuroprotective treatments.
Particular to glaucoma, there is also an unmet clinical need for a surrogate marker to predict future disease progression. Results from the Phase II study should be reported soon.
 |
| Fig. 2. This retinal image was acquired using the DARC technique during the DARC Phase II trial. The individual apoptosing retinal nerve cells (bright spots) are clearly distinguishable. |
Future Practice Implications
DARC offers a unique imaging technique that is able to use the eyes as a ‘window’ into the central nervous system in order to characterize events at a cellular level. This novel technique could potentially provide a powerful new tool to identify patients with pre-perimetric glaucoma, enabling early treatment, which may prevent or delay irreversible visual loss.
Just enabling early therapy initiation by conventional pressure-lowering means would be a benefit we hope to see DARC bring to light. But the treatment effect of neuroprotective agents, such as brimonidine, memantine and glutamate, can all be documented via DARC imaging. The quest to achieve a viable neuroprotective treatment is a Herculean task, and we hope our efforts to develop DARC might assist in the undertaking.
This new technology platform also opens the possibility of directly observing the effect of treatments in neurodegenerative disease using an endpoint based on the direct assessment of retinal cell death, therefore serving as a surrogate biomarker in clinical trials.
The hope is that with the results from further work in other conditions, DARC may also improve diagnosis and provide treatment options in a variety of neurodegenerative conditions, for which there are no effective cures.
These are early days for sure, but we on the research team are optimistic and excited about where we’re heading.
Ms. Almonte is the research nurse at Imperial College Ophthalmic Research Group, Imperial College London.
Dr. Cordeiro is professor of glaucoma and retinal neurodegeneration studies at University College London, professor of ophthalmology and director of the ophthalmology research group at Imperial College London, and consultant ophthalmologist at Western Eye Hospital.
| 1. Kerrigan-Baumrind LA, Quigley HA, Pease ME, et al. Number of ganglion cells in glaucoma eyes compared with threshold visual field tests in the same persons. Invest Ophthalmol Vis Sci. 2000;41(3):741-8. 2. Zeyen T. Target pressures in glaucoma. Bull Soc Belge Ophtalmol. 1999;274:61-5. 3. Oliver JE, Hattenhauer MG, Herman D, et al. Blindness and glaucoma: a comparison of patients progressing to blindness from glaucoma with patients maintaining vision. Am J Ophthalmol. 2002;133(6):764-72. 4. Congdon N, O’Colmain B, Klaver CC, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122(4):477-85. 5. Gupta P, Zhao D, Guallar E, et al. Prevalence of glaucoma in the United States: The 2005-2008 National Health and Nutrition Examination survey. Invest Ophthalmol Vis Sci. 2016;57(6):2905-13. 6. Selleri C, Maciejewski JP, Sato T, Young NS. Interferon-gamma constitutively expressed in the stromal microenvironment of human marrow cultures mediates potent hematopoietic inhibition. Blood. 1996;87(10):4149-57. 7. Raza A, Mundle S, Iftikhar A, et al. Simultaneous assessment of cell kinetics and programmed cell death in bone marrow biopsies of myelodysplastics reveals extensive apoptosis as the probable basis for ineffective hematopoiesis. Am J Hematol. 1995;48(3):143-54. 8. Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267(5203):1456-62. 9. Festoff BW. Amyotrophic lateral sclerosis: current and future treatment strategies. Drugs. 1996;51(1):28-44. 10. Pollard H, Cantagrel S, Charriaut-Marlangue C, et al. Apoptosis associated DNA fragmentation in epileptic brain damage. Neuroreport. 1994;5(9):1053-55. 11. Portera-Cailliau C, Sung CH, Nathans J, Adler R. Apoptotic photoreceptor cell death in mouse models of retinitis pigmentosa. Proc Natl Acad Sci USA. 1994;91(3):974-78. 12. Gschwind M, Huber G. Apoptotic cell death induced by beta-amyloid 1-42 peptide is cell type dependent. J Neurochem. 1995;65(1):292-300. 13. Walkinshaw G, Waters CM. Induction of apoptosis in catecholaminergic PC12 cells by L- DOPA. Implications for the treatment of Parkinson’s disease. J Clin Invest. 1995;95(6):2458-464. 14. Reme CE, Grimm C, Hafezi F, et al. Apoptotic cell death in retinal degenerations. Prog Retin Eye Res. 1998;17(4):443-64. 15. Yap TE, Donna P, Almonte MT, Cordeiro MF. Real-time imaging of retinal ganglion cell apoptosis. Cells. 2018;7(6). 16. Galvao J, Davis BM, Cordeiro MF. In vivo imaging of retinal ganglion cell apoptosis. Curr Opin Pharmacol. 2013;13(1):123-27. |

