 A 62-year-old white female presented to the office as a new patient on referral from a mutual friend. She wanted to learn if she was a good candidate for LASIK surgery. She took no medications and reported no known allergies. While her general health was unremarkable, she had a family history of both Alzheimer’s and Parkinson’s disease. She last visited her previous eye doctor in 2007.
A 62-year-old white female presented to the office as a new patient on referral from a mutual friend. She wanted to learn if she was a good candidate for LASIK surgery. She took no medications and reported no known allergies. While her general health was unremarkable, she had a family history of both Alzheimer’s and Parkinson’s disease. She last visited her previous eye doctor in 2007.
Diagnostic Data
Her uncorrected visual acuity measured 20/100 O.D. and O.S. She wore OTC spectacles for reading only, and she had driven to the office uncorrected. Her best-corrected visual acuity measured 20/20 O.D. and O.S. through a refraction of +2.50 O.D. and +2.75 O.S. We determined her add to be +2.00 O.U., resulting in a measurement of J1 O.D. and O.S.
Her pupils were equally round and reactive to light and accommodation, with no afferent defect. Confrontation fields were full to finger counting O.U., and her extraocular muscles were full in all diagnostic positions of gaze O.U.
A slit lamp examination revealed the presence of narrow angles (grade I O.U. by Van Herick’s method). Her IOP was 13mm Hg O.D. and 15mm Hg O.S. at 5:00 p.m. Central corneal thickness measured 544µm O.D. and 561µm O.S. Gonioscopy revealed grade I angles 360° O.U., with slightly wider angles nasally in each eye. So, we performed dynamic gonioscopy with a Posner four-mirror lens, which easily opened the angles. Threshold visual fields were normal O.U.
Through dilated pupils, her crystalline lenses were characterized by early incipient nuclear sclerosis. She had vitreous floaters O.U. Following dilation, her IOP measured 16mm Hg O.D. and 14mm Hg O.S.
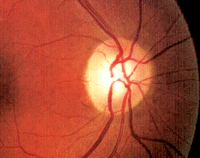
It can be difficult to identify early glaucomatous damage in patients with larger optic nerves, as seen here.
Stereoscopic examination of her optic nerves revealed a cup-to-disc ratio of 0.55 x 0.60 O.D. and 0.40 x 0.45 O.S. Her optic nerves appeared somewhat large on slit lamp examination.
Her macular evaluation was completely normal O.U., as was her peripheral retinal exam. Vascular evaluation showed no abnormalities.
Discussion
Though our patient originally presented to determine if she could undergo LASIK, I quickly became interested in her narrow angles and asymmetric cups. I informed her that she was not an ideal LASIK candidate due to her hyperopia. She seemed to take the news quite well, suggesting that her previous eye care provider had expressed the same concern.
Then, given her angle and cup appearance, we proceeded to discuss her potential risk for glaucoma. I asked her if she had been told previously that she was at risk for glaucoma development––she replied, “No.”
So, how would you proceed to manage this patient? Would she be best served by in-office monitoring, or should you refer her for a laser iridotomy?
While this question could be debated from both perspectives, you must first answer one fundamental question: Does the patient have narrow-angle glaucoma, or is she simply at risk?
If she does indeed have narrow-angle glaucoma, you must intervene. But remember, the presence of narrow angles does not automatically imply that she has glaucoma.
Given the asymmetric appearance, you may initially assume that she has optic nerve damage––especially in the right eye. However, a closer examination on Heidelberg Retinal Tomography-3 (HRT-3, Heidelberg Engineering) confirmed that her optic nerves are rather large (O.D.>O.S.). We know that larger optic nerves typically have larger optic cups. So, in this case, it was safe to associate her large cups with large nerves. In other words, I did not believe that she currently exhibited any glaucomatous damage.
With this in mind, we no longer had to worry about using laser iridotomy to treat narrow-angle glaucoma, but instead, could safely consider employing it to reduce her risk for narrow-angle glaucoma development—an important distinction.
There are multiple risk factors for developing angle closure glaucoma, including medication use, anterior chamber anatomy and hyperopia.1-3 Treatment options can include laser iridotomy, iridectomy and/or cataract surgery.4,5
Do all narrow angles require intervention? Depending on the practice setting, one clinician may choose to proceed with surgical prophylaxis, and another may not.
In our case, because our patient was merely at risk with narrow but open angles, I believe it was acceptable simply to observe the patient for structural or functional changes to her optic nerves over a period of time. Should any changes occur, I would recommend interventional prophylaxis.
In the meantime, we have established adequate baseline information and have scheduled her for a one-year follow-up. However, we instructed her to contact us immediately if she develops any symptoms consistent with angle closure, including ocular or facial pain; unilateral vision blur; or unexplained nausea or vomiting.
1. Tahiri Joutei Hassani R, Dupont Monod S, Oukacha G, et al. [Acute bilateral angle-closure glaucoma induced by topiramate: Contribution of Visante((R)) OCT.] J Fr Ophtalmol. 2010 Apr 28. [Epub ahead of print]
2. Aptel F, Denis P. Optical coherence tomography quantitative analysis of iris volume changes after pharmacologic mydriasis. Ophthalmology. 2010 Jan;117(1):3-10.
3. Quigley HA. Angle-closure glaucoma-simpler answers to complex mechanisms: LXVI Edward Jackson Memorial Lecture. Am J Ophthalmol. 2009 Nov;148(5):657-69.e1.
4. Lachkar Y. [Acute angle closure and angle closure glaucoma: Phacoemulsification as first-line treatment] J Fr Ophtalmol. 2010 Apr;33(4):273-8.
5. Tham CC, Kwong YY, Leung DY, et al. Phacoemulsifi-cation vs phacotrabeculectomy in chronic angle-closure glaucoma with cataract complications. Arch Ophthalmol. 2010 Mar;128(3):303-11.

