Glaucoma surgery has entered a renaissance period. In recent years, emerging technologies for microinvasive glaucoma surgery (MIGS) have set the stage for revolutionary approaches that target patients with mild to moderate glaucoma. This modern era of glaucoma surgery has clearly placed improved patient safety at the center of innovation.
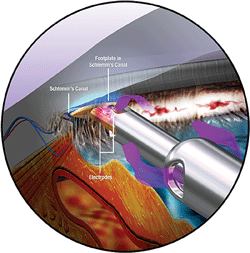
1.Triangular configuration of the handpiece tip, which is inserted through the trabecular meshwork into Schlemm’s canal. Photo: NeoMedix
MIGS is a term that encompasses a wide variety of new instruments and techniques that are rapidly being adapted by ophthalmic surgeons. Examples of FDA-approved MIGS devices include Trabectome (NeoMedix Corporation) and the iStent Trabecular Micro-Bypass Stent (Glaukos Corporation). Other MIGS technologies include the Ex-Press Glaucoma Filtration Device (Alcon Laboratories), the AquaSys Implant (AquaSys) and the CyPass MicroStent (Transcend Medical).
The Trabectome instrument has been available the longest, receiving FDA approval back in 2004. Additionally, the iStent just received FDA clearance in 2012.
Glaucoma Surgery 101
The lowering and stabilization of intraocular pressure (IOP) is the only proven treatment for glaucoma.1,2 Contemporary glaucoma surgery decreases IOP in the eye either by inhibiting aqueous production or by enhancing its outflow.
An example of the former is endoscopic cyclophotocoagulation (ECP), where energy from a diode laser is directly applied to the ciliary processes that produce the aqueous in the eye (see “
Consider ECP for Glaucoma,” November 2008).
Most of the other glaucoma surgeries enhance aqueous outflow. The procedures that do this can be divided into those that form a bleb (extra-scleral drainage) and those that enhance physiologic outflow (“ab interno”).
• Bleb surgery. The bleb-forming incisional glaucoma surgeries lower IOP by creating a direct communication between the anterior chamber and subconjunctival space. This procedure completely bypasses the conventional route of aqueous drainage through the trabecular meshwork, Schlemm’s canal and the collecting channels.
The subconjunctival fluid collects focally within the bleb and the aqueous resorbs into the episcleral venous system.3 The two classic bleb-forming surgeries are trabeculectomy and glaucoma drainage implantation (tube shunts).
• Ab interno surgery. This surgical approach, on the other hand, eliminates the need for conjunctival dissection––thus, there is no bleb formation. The procedure either removes the trabecular meshwork and inner wall of Schlemm’s canal or bypasses these two structures–– effectively re-establishing aqueous outflow directly into the collecting channels.
This direct access to the collecting channels is significant because the trabecular meshwork is the anatomic location that poses the greatest resistance to aqueous outflow.4 Examples of ab interno glaucoma surgeries include Trabectome and iStent, both of which are MIGS procedures.
Trabectome Instrumentation
The Trabectome consists of a 19.5-gauge insulated handpiece that fits through a 1.7mm corneal incision. The handpiece is connected to a console with irrigation and aspiration, and a high-frequency electro-surgical generator. The handpiece’s triangular, sharp point assists in its insertion through the trabecular meshwork and into Schlemm’s canal (figure 1).
When the instrument is turned on, the trabecular meshwork is selectively vaporized and aspirated by a series of energy pulses. Rather than cause thermal ablation, the bursts disrupt and disintegrate the tissue (figure 2).5,6
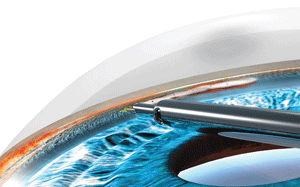
2. The trabecular meshwork and inner wall of Schlemm’s canal are selectively vaporized and aspirated using high-frequency bursts of electrocautery heat energy. Photo: NeoMedix
A 90° to 120° strip of trabecular meshwork tissue is removed via one surgical incision (figure 3). (Removal of more than 120° of tissue requires two surgical incisions into the cornea.)
The irrigation, aspiration and electro-surgical functions are controlled by the surgeon’s foot pedal. The initial power setting, which also is controlled by the surgeon, ranges from 0.7 watts to 0.9 watts.7,8
The tip of the handpiece is very sharp and must not touch the iris during its advancement in the anterior chamber, because consequential bleeding would obscure the image and potentially force the surgeon to abort the procedure.
Trabectome Surgery
If the trabectome surgery is being performed in combination with cataract extraction, the typical preoperative dilating, non-steroidal anti-inflammatory drug and antibiotic regimen is indicated.8 In the standalone trabectome surgery, however, 1% or 2% pilocarpine is administered to yield pupillary miosis for easier instrument access into the chamber angle.3
Viscoelastic is injected into the anterior chamber and the handpiece is advanced towards the opposite chamber angle. This almost always is the nasal angle because the clear corneal incision will be placed temporally, which makes the trabecular meshwork accessible to the surgeon. Visualization of the chamber angle is achieved with a goniosurgical lens (a modified Swan-Jacobs lens) that is placed on the cornea (figure 4).

3. A strip of 90° to 120° of tissue usually is removed via one surgical incision. Photo: NeoMedix
An interesting aspect of this surgery is the positioning of both the patient and the surgeon. The patient’s head is rotated away from the surgeon. This helps expose the temporal approach to the cornea.
By rotating the surgical microscope 45°, the nasal chamber angle will be revealed with gonioscopic viewing. The overall combined angle from the patient’s eye in primary gaze is approximately 70° to 80°. (This positioning reminds me of how a rider would sit on a chopper motorcycle.)
After the trabecular material is removed, the surgical incision is closed with a suture to ensure a leak-proof wound.9 The patient is placed on routine postoperative medications as well as 1% pilocarpine TID to QID for at least one month. The pilocarpine helps keep the iris tissue out of the angle, minimizes contraction of the surgical opening and limits scar tissue formation.
• Postoperative complications. Trabeculectomy surgery and glaucoma drainage implants carry a higher risk of postoperative complications than most MIGS procedures, including Trabectome. Trabeculectomy has a 1% per year risk of endophthalmitis and other frequent vision-threatening outcomes, such as persistent hypotony, choroidal detachments, aqueous misdirection (malignant glaucoma), blebitis and bleb leak.10,11 Drainage implants can cause complications, such as motility disturbances, hypotony, corneal decompensation and tube erosions.12
The most frequent complications seen with Trabectome are transient hyphema and elevated IOP at one-day post-op.3 Bleeding at the surgical site occurs routinely during the removal of trabecular meshwork tissue with this procedure.
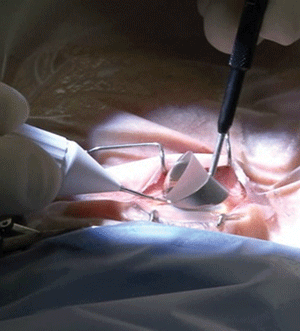

4, 5. Surgical positioning
of the Trabectome handpiece within the eye and the goniosurgical lens
on the cornea (left). Gonioscopy shows a wide-open surgical cleft one
month after Trabectome surgery. Photos: NeoMedix
Additionally, postoperative cataract development is less likely following Trabectome than after other bleb-forming surgeries (unless there is inadvertent perioperative lens damage).
Hypotony also is rare because the corneal incision is closed with a suture. Over time, the chamber angle trabecular meshwork may undergo scarring and close the cleft, causing an IOP increase (figure 5).3
• Success rate. The anticipated postoperative IOP range associated with Trabectome surgery is in the mid teens.3 Results from a retrospective review of 1,127 Trabectome procedures (738 Trabectome-only and 366 Trabectome-phacoemulsification surgeries) show an IOP reduction in Trabectome-phacoemulsification of 18% at 12 months, and a 40% reduction for Trabectome-only at 24 months.13
Additionally, other studies have indicated that patients use 33% to 40% less topical glaucoma medication for up to two years postoperatively.3,6
iStent Technology
Similar to the Trabectome, implantation of the iStent is an ab interno procedure that fundamentally eliminates the need for conjunctival dissection.
Glaucoma surgeons have learned that implanting larger drainage devices does not always yield a better result. Based on this lesson, the iStent is the smallest medical device ever implanted into humans—less than 1mm in length and 0.5mm in width (figure 6).14
The titanium stent is self-trephining and is implanted using an injector. After the device is implanted into Schlemm’s canal via the trabecular meshwork, the greatest resistance to aqueous outflow is bypassed.

6. The iStent is the smallest medical device ever implanted into humans. Photo: Glaukos
Just like with Trabectome surgery, direct visualization of the chamber angle and its structures is achieved via a goniosurgical lens and an operating microscope. A stable anterior chamber is important in the early postoperative period. Therefore, as with Trabectome surgery, a suture may need to be placed through the corneal surgical incision.
• Success rate. Schlemm’s canal is not a continuous chamber that encircles the eye 360° degrees. Instead, the canal has 20 to 35 collector channels, and an iStent typically accesses only one or two of these.14 So, more than one stent may need to be implanted to achieve additional IOP lowering.
Combined cataract surgery and iStent implantation also can be performed. Usually, the cataract surgery is done before the iStent placement. In a one-year prospective trial, 240 eyes with mild to moderate glaucoma were randomly assigned to cataract surgery and iStent implantation (stent group) or cataract surgery alone (control group).15 The researchers found that a significantly higher proportion of patients in the stent group (72% vs. 50%) achieved a postoperative IOP measurement of less than 21mm Hg without medication.15
A two-year safety and efficacy follow-up study showed similarly favorable results.16 Sixty-one percent of patients who underwent stent implantation achieved an IOP measurement of 21mm Hg or lower without the use of ocular hypotensive medications, compared to 50% of patients who underwent cataract surgery alone.
With the advent of the new MIGS procedures, all eye care clinicians should experience a welcome shift in the treatment of glaucoma patients. Gone will be the days of patients needing to use three or even four different pressure-lowering medications. Also gone will be waiting until the disease has advanced far enough to warrant a more aggressive and complicated extra-scleral drainage surgery.
Because of the increased safety associated with MIGS procedures, as well as their efficacy in mild to moderate glaucoma and ease of surgical application, more and more anterior segment surgeons will be incorporating these techniques during the next few years.
Dr. Skorin is the senior staff ophthalmologist at Mayo Clinic Health System in Albert Lea, Minn., and is an assistant professor of ophthalmology at the Mayo Clinic College of Medicine. He is certified in Trabectome, but has no direct financial interest in any of the products mentioned.
1. Heijl A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression: Results from The Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002 Oct;120(10):1268-79.
2. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. The AGIS Investigators. Am J Ophthalmol. 2000 Oct;130(4):429-40.
3. Mosaed S, Rhee DJ, Filippopoulos T, et al. Trabectome outcomes in adult open-angle glaucoma patients: One-year follow-up. Clin Surg Ophthalmol. 2010;28(8/9):182-6.
4. Fleischman D, Berdahl JP. Surgical anatomy. In: Kahook MY (ed.). Essentials of Glaucoma Surgery. Thorofare, NJ: Slack Incorporated; 2012:11-22.
5. Francis BA, See RF, Rao NA, et al. Ab interno trabeculectomy: Development of a novel device (Trabectome) and surgery for open-angle glaucoma. J Glaucoma. 2006 Feb;15(1):68-73.
6. Francis BA. Trabectome combined with phacoemulsification versus phacoemulsification alone: A prospective, non-randomized controlled surgical trial. Clin Surg Ophthalmol. 2010;28(10/11):228-35.
7. Francis BA, Minckler D, Dustin L, et al. Combined cataract extraction and trabeculotomy by the internal approach for coexisting cataract and open-angle glaucoma: initial results. J Cataract Refract Surg. 2008 Jul;34(7):1096-103.
8. Horsley MB, Rhee DJ: Combined phacoemulsification and Trabectome. In: Kahook MY (ed.). Essentials of Glaucoma Surgery. Thorofare, NJ: Slack Incorporated; 2012: 149-52.
9. Vold SD, Dustin L. Impact of preoperative intraocular pressure on Trabectome outcomes: A prospective, non-randomized, observational, comparative cohort outcome study. Ophthalmic Surg Lasers Imaging. 2010 Jul-Aug;41(4):443-51.
10. Eisengart JA. Trabeculectomy: Management of postoperative complications. In: Kahook MY (ed.). Essentials of Glaucoma Surgery. Thorofare, NJ: Slack Incorporated; 2012: 49-58.
11. Greenfield DS, Suñer IJ, Miller MP, et al. Endophthalmitis after filtering surgery with mitomycin. Arch Ophthalmol. 1996 Aug;114(8):943-9.
12. Eisengart JA. Glaucoma drainage devices: Management of intraoperative and postoperative complications. In: Kahook MY (ed.). Essentials of Glaucoma Surgery. Thorofare, MJ: Slack Incorporated; 2012:121-31.
13. Minckler D, Mosaed S, Dustin L, Ms BF. Trabectome (trabeculectomy-internal approach): additional experience and extended follow-up. Trans Am Ophthalmol Soc. 2008;106:149-59; discussion 159-60.
14. Berdahl JP. Cataract extraction plus iStent. In: Kahook MY (ed.). Essentials of Glaucoma Surgery. Thorofare, NJ: Slack Incorporated; 2012:171-5.
15. Samuelson TW, Katz LJ, Wells JM, et al. Randomized evaluation of the trabecular micro-bypass stent with phacoemulsification in patients with glaucoma and cataract. Ophthalmology. 2011 Mar;118(3):459-67
16. Craven ER, Katz LJ, Welss MW, et al. Cataract surgery with trabecular micro-bypass stent implantation in patients with mild-to-moderate open-angle glaucoma and cataract: two-year follow-up. J Cataract Refract Surg. 2012 Aug;38(8):1339-45.

