Visual field testing is an essential part of eye care, necessary for diagnosis and management of multiple conditions we see on a daily basis. Perimetry is subjective in nature, and it is necessary to take care in both the acquisition and analysis of the testing data. Many barriers to successful visual field testing exist, but much of the frustration encountered can be avoided by following some basic guidelines and using all the technological features today’s devices offer.
1. Pick the right test
Most visual field testing is “standard automated perimetry” (SAP). SAP is a computerized, threshold static perimetry that tests the central visual field with a white stimulus on a white background. Threshold testing has been the standard for glaucoma care since the mid 1980s, offering many advantages over the older standard of manual kinetic perimetry.1-2
SAP test patterns can be categorized as threshold or screening. Common threshold patterns are 10-2, 24-2, 30-2 and 60-4. Field analysis in glaucoma relies primarily on the 24-2 and 30-2 patterns, as the majority of ganglion cells lie within the central 30 degrees of fixation.3 Use of 24-2 has become increasingly prevalent as the test of choice in glaucoma due to its faster testing time and reduced trial lens and lid artifact errors. A 24-2 has 54 test points and is identical to the 72-point 30-2 testing protocol except for the removal of most outer ring test points (24-2 retains the two outermost nasal points from the 30-2 pattern).
Both of these patterns have test points spaced six degrees apart. Points straddle the mid-line, allowing for better identification of glaucomatous defects.
Swedish Interactive Thresholding Algorithm (SITA) Standard in 24-2 pattern with stimulus size III is generally the preferable test for most routine glaucoma and neurological testing.4 Clinicians often have the misconception that SITA Fast strategy is an easier test for patients who have difficulty taking a SITA Standard or full threshold strategy test. SITA Fast does take 2-5 minutes per eye to perform (compared with 3-7 minutes per eye for SITA Standard). However, the algorithm it uses presents points requiring more discretion from the patient, and it is best used in experienced test takers or young patients.5
2. Know when to modify the testing strategy
Stimulus size III is standard for most situations and should be used in patients with 20/200 or better. Increase size to V in patients with poorer vision (this may be indicated in some patients with advanced glaucoma). When altering the stimulus, keep in mind that the normative database, SITA test strategy, and progression analysis will no longer be available. When severe field loss in advanced glaucoma is present, change to a 10-2 pattern to allow for more accurate assessment of the remaining visual field. In cases where vision is reduced due to macular disease or central scotoma, use a diamond fixation target—this displays four LEDs, allowing the patient to center their gaze between the targets.6
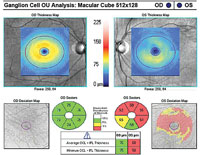 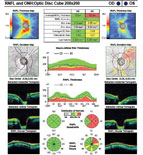 | |
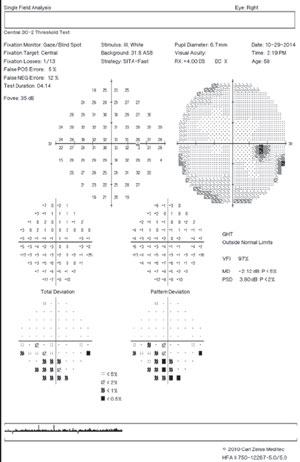
| Fig. 1. A 10-2 visual field can be a valuable addition to standard 24-2 and 30-2 testing. Alternating these tests will allow for better detecting of defects that threaten fixation. This 58-year-old black male has early normal-tension glaucoma associated with extensive macular ganglion cell loss and a visual field defect that surrounds fixation. BCVA was 20/20 OD and 20/25 OS. | |
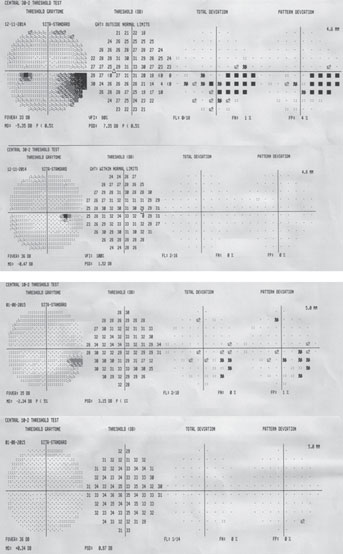 |
3. Don’t overlook the central field in early glaucoma
The macula is +/-8 degrees from fixation and represents only a small portion of the retinal area, but contains about 30% of the retinal ganglion cells.3 A 24-2 test only includes four points within this highly sensitive region. The 24-2 test protocol was designed to detect nasal and arcuate glaucomatous defects. These defects originate from damage to the arcuate nerve fiber bundles—often visible on funduscopy with retinal nerve fiber layer (RNFL) wedge defects or neuroretinal rim notching.
Research shows significant sections of the inferior macula are associated with the inferior arcuate bundle (the majority of macular ganglion cells associated with the temporal quadrant of the optic nerve which is often preserved in early glaucomatous disease).7
If there is even a single central point defect with a 24-2 or 30-2 pattern, consider adding a 10-2 test to your assessment (figure 1). This will allow proper detection of early macular damage. The inferior temporal quadrant of the circumpapillary RNFL is particularly susceptible to this damage and will correlate with a small arcuate defect in superior central visual field. This 35-degree region of the RNFL has been termed the “macular vulnerability zone.”7
Also, be aware that macular damage can present diffusely rather than sectorally.
4. Understand the roles of the technician and physician
It’s important that the staff and physician maintain positive attitudes about the value of perimetry to encourage the patient to provide optimal results during testing.8 Although the physician should not administer the test personally, they should take time during the exam to stress to the patient the importance of perimetry in the management of their ocular disease. Physicians should continue to encourage patients when subsequent testing is ordered or reviewed.
Technicians should periodically get a refresher on the importance of perimetry. Those performing perimetry should take the test themselves, so they can more effectively explain it to patients. Technicians should always be present during the testing period so they may provide re-education, as necessary, and feedback regarding testing reliability. A technician who does not properly respond to patients’ perimetry complaints promotes poor test taking.
5. Recognize, reduce artifacts
Peripheral points, particularly in a 30-2 test, are susceptible to variability and artifact. Trial lens artifacts usually produce sharp depressions at peripheral points, often in a ring pattern. These artifacts are more common in moderate-high hyperopic corrections and when two trial lenses are used. Make certain the lens is placed as close to the eye as possible; also, using spherical equivalent up to 2.00D of refractive cylinder will help reduce some of these errors.
Pupil size smaller than 2mm or larger than 6mm can induce artifacts. If you decide to dilate a patient due to miosis, make certain to remain consistent on subsequent testing. If ptosis or dermatochalasis produce obstruction of the superior field, then the eyelids may be taped for testing (again, this should be noted on the field report to maintain consistency on all testing).
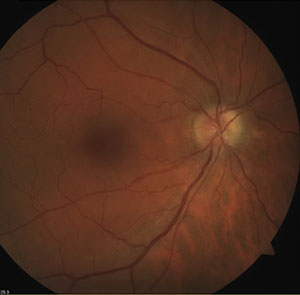 | |
| Fig. 2. Optic disc drusen may produce visual field defects similar to those seen in glaucoma. This 58-year-old white male was found to have an inferior visual field defect correlating to superior disc drusen (evident in the fundus photo). | |
 |
6. Interpreting results systematically
Don’t take shortcuts in reviewing data from visual fields—the professional component of testing is the interpretation, and each analysis report contains a wealth of data.
Know if a test is reliable; fixation losses greater than 20% can indicate poor reliability, but improper mapping of the blind spot can cause false elevation of this index. Gaze tracking allows for more accurate interpretation of patient fixation stability. Gaze tracking measures up to one degree, whereas traditional fixation monitoring is sensitive for three degrees (half the size of the physiologic blind spot).
False positives are a key reliability index. “Trigger happy” patients will push the response button in the absence of a stimulus. False positives are primarily important in tests that have defects—they are not a reason to invalidate an otherwise unremarkable or clear visual field test. If this index is higher than 15%, the test needs to be invalidated or repeated (even 5% to 10% should be scrutinized).Tests with high false positives are automatically removed from a Humphrey Guided Progression Analysis (GPA).
False negatives represent variability in patient responses and are seen at increasing levels in depressed visual fields. A false negative value of 10% to 15% or more is suggestive of inattention in a patient without a significant field defect. If significant glaucomatous loss is present, false negatives should not deem a test unreliable if it otherwise appears reliable.9
Age matters—the Humphrey database uses norms grouped by age in 10-year intervals. Keep in mind that a patient’s results may appear to improve due to this grouping effect. For instance, if testing was performed at age 59 and, on subsequent examinations, age 60, the second test would be compared with a different database than the first test.
Know if you are detecting or monitoring a defect. Your interpretation strategy should differ in patients being evaluated for the presence of a glaucomatous defect and patients who have established visual field loss.
In patients who are glaucoma suspects, rely on the Glaucoma Hemifield Test (GHT) and the pattern deviation probability map. GHT was designed to have high sensitivity and specificity for glaucomatous defects.10 It uses five zones in each hemifield and tests them for symmetry based on a normative database.
GHT outside normal limits is displayed when at least one zone is at p <1%. This finding has been show to have 94% specificity.11 GHT borderline is displayed when the p value is between 1% and 3%. Generalized depression indicates that the most sensitive test points are less than 50% of normal. Abnormally high sensitivity is a red flag and indicates low reliability (usually associated with a high rate of positives).
If a field shows scattered non-specific depressions, keep in mind that a diagnostic early glaucomatous defect is generally recognized as a repeatable cluster of three or more points on the same side of the horizontal meridian all reaching statistical significance (with one or more points having p<1% significance)—the test points adjacent to the blind spot may be ignored.
If a patient with visual field loss is being monitored with serial examination, use the mean deviation (MD), pattern standard deviation (PSD) and visual field index (VFI) to track progression.
MD is sensitive to media opacity, uncorrected refractive error, and miosis. MD will be less sensitive in early-stage glaucoma and other cases with localized field loss.
The GHT can better aid in identifying early, localized defects, and PSD can be useful for tracking these milder defects. In the presence of other suspicious findings, a PSD of p <5% is a strong indicator of glaucomatous loss. PSD, however, will spike in early disease and peak in the early part of advanced disease; however, it will then however decrease due to reduced overall field sensitivity (worsening cataract may also decrease PSD).
VFI is a metric that was created to help with staging and progression of glaucoma. It is intended to reflect ganglion cell loss and function. VFI is displayed in a percentage from 0 to 100. It is weighted preferentially to central points and more resistance to cataract.12
7. Test, test, repeat
The real utility of visual fields lies in tracking progression of glaucomatous defects. The European Glaucoma Society (EGS) recommends visual field testing several times yearly for the first two years after diagnosis.13 This will help establish a rate of progression and identify the roughly one in six glaucoma patients who progress at a dangerously high rate (greater than 2dB per year).14 If this frequency is not reasonable in your practice setting, then test at least twice yearly during the first two years. Testing frequency can decrease at the two-year and five-year marks once a progression rate is reliably established.
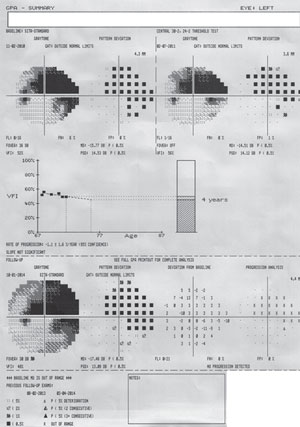 | |
| Fig. 3. The GPA summary report will show two baseline fields: a Glaucoma Change Probability Map (an event analysis) of the most current test and a VFI graph (trend analysis) for all available fields. This is a patient with advanced glaucoma and progressing cataract. No progression is detected adjacent to the dense arcuate scotoma, which itself is too depressed to allow for progression analysis. |
Also, keep in mind that an artifactual reduction in sensitivity may be seen on the first perimetric test in approximately 10% of patients.15
These patients may require two or three tests to produce an accurate and reliable baseline result.
8. Be on the look out for masquerading retinal and optic nerve conditions
Concomitant retinal or neurological disease can confound interpretation of visual field defects in many patients. Altitudinal or arcuate field defects may be seen in anterior ischemic optic neuropathy, vascular occlusion, optic disc drusen (figure 2) or sectoral retinal photocoagulation treatment. Peripheral field constriction may be present in optic neuritis, nonglaucomatous optic atrophy, advanced retinitis pigmentosa or acute zonal occult ocular retinopathy (AZOOR). Nonproliferative diabetic retinopathy can also cause scattered visual field deviations, even in mild stages.16 Moderate and severe diabetic retinopathy will more likely have a dense and repeatable visual field defect.
9. Use progression analysis tools
We expect progression in the majority of glaucoma patients. In fact, the Early Manifest Glaucoma Treatment Trial showed that 59% of glaucoma patients will progress in eight years, even if treated and well controlled.17 Determining if the rate of progression will affect visual function and quality of life is important when making the decision to proceed with escalating therapy that carries increased risk of side effect. Even in light of 21st century technology, serial visual field testing remains the most accurate means of determining progression in glaucoma. Modern perimeters are equipped with powerful software tools that allow practitioners to accurately track these metrics.
The best tools for progression analysis on HFA units are GPA Change Probability, VFI trend, and MD trend, and linear regression and cluster regression analysis on Octopus units. GPA requires a minimum of five tests to fully utilize its features. Two tests will be selected automatically for baseline, but these tests may be manually selected. From this baseline, GPA is able to provide both event- and trend-based progression analysis on future tests (figure 3).
Event-based progression determines whether or not progression has occurred on a point-by-point basis. Trend-based progression will determine the rate of progression. Event-based progression analysis has been used in several landmark glaucoma clinical trials (such as EMGT, AGIS and CIGTS).18-20 The GPA Change Probability Map displays a point-by-point analysis and indentifies progression if at least three points have worsened (marked as “possible progression” if repeated in two tests and “likely progression” if repeated in three tests). These criteria for progression were established by the EMGT.18 Using this method has 96% sensitivity in 30-2 and 91% in 24-2. Keep in mind that detecting progression often takes two or more years. EMGT had a mean of 33 months to progression in 30-2 protocols and 37 months for 24-2.5
Trend-based progression will display linear regression analysis of VFI. If the tests span two or more years, the software will plot a future prediction of progression. Prior to VFI, MD was used for progression analysis, but VFI provides a more accurate determination in the presence of cataract and cataract surgery.21
If a patient experiences non-glaucomatous loss due to vascular occlusion, ischemic optic neuropathy or panretinal laser, it is necessary to establish a new baseline for visual field testing as well as change the tests used in progression analysis. Make certain to check the two baseline tests in GPA for accuracy. Any tests are usable unless false positives are higher than 15%. Avoid using fields that are further than 6-12 months apart for baseline. Update to new baselines if there is a significant change in therapy (such as filtration procedure). Also note that GPA is not available when MD is over 20dB.
Using progression analysis for nonglaucomatous field loss should be limited to VFI linear regression/trend analysis (the GPA change probability was designed for use in glaucoma). Some conditions, such as optic neuritis, may have such a high inherent variability that quantitative progression analysis is not possible. For neurological field loss, the overview report is preferred.
10. Make the correlation between structure, function
Conventional wisdom holds that structural change precedes functional loss in glaucoma. However, we often see patients who demonstrate significant RNFL loss prior to repeated visual field defects as well as patients progressing on their fields without detectable progression of RNFL or ganglion cell loss. Clinicians should nonetheless seek to find correlation of structure and function to help strengthen diagnosis and bring attention to specific areas in complementary testing components.
Understand that early glaucomatous defects may have variable depth and location (although they will be in the same area). The defect will deepen into a repeatable defect with time. The nasal and superior fields are more likely to show early glaucomatous defects.
Extensive RNFL loss (~30%) is necessary to produce a visual field defect.22,23 An individual (especially a patient with a large optic nerve) may have as much as one third of her or his RNFL deteriorate while still maintaining “green” normative levels on OCT analysis (this is often referred to as “green disease”).24 A recent study reported the highest correlation between progression on OCT and visual field to occur at a 10µm RNFL loss on SD-OCT.25 For many patients, advanced glaucomatous optic atrophy will result in a severely depressed RNFL that does not allow for proper detection of progression. For these patients in particular, visual field testing will best provide information about the progression of their disease.
Having an idea of the patient’s independent risk factors for glaucoma, aside from structural and functional testing, can allow clinicians to either raise or lower their threshold for diagnosis. If a patient is high risk given IOP and age, then a structure and function correlation is certainly not necessary to establish good certainty of a diagnosis. Conversely, you would hold testing on a healthy 40-year-old patient to a high degree of specificity—the condition is much less commonly seen in that population and the diagnosis carries with it the potential of significant burden due to the long life expectancy.
Dr. Horton is a staff optometrist at the Cincinnati VA Medical Center. He is also an adjunct faculty member at Ohio State University College of Optometry.
1. Heijl A. Automatic perimetry in glaucoma visual field screening. A Clinic Study. Albrecht Von Graefes Arch Klin. Exp Ophthalmol. 1976;200(1):21-37.2. Herbolzheimer W. Computer program controlled perimetry, its advantages and disadvantages. Klin Monbl Augenheilkd. 1986;189(4):270-7.
3. Hebel R, Hollander H. Size and distribution of ganglion cells in the human retina. Anatomy and Embryology. 1983;168(1):125-136.
4. Khoury J, Donahue S, Lavin P, Tsai J. Comparison of 24-2 and 30-2 perimetry in glaucomatous and nonglaucomatous optic neuropathies. J Neuroophthalmol. 1999;19(2):100-8.
5. Heijl A, Bengtsson B, Chauhan B, et al. A comparison of visual field progression criteria of 3 major glaucoma trials in early manifest glaucoma trial patients. Ophthalmology. 2008;115(9):1557-65.
6. Humphrey Field Analyzer User Manual. Dublin, CA: Carl Zeiss Meditec, Inc.; 2010.
7. Hood D, Raza A, de Moraes C, et al. Glaucomatous damage of the macula. Prog Retin Eye Res. 2013; 32: 1-21. doi: 10.1016/j.preteyeres.2012.08.003. Epub 2012 Sep 17.
8. Kutzko K, Brito C, Wall M. Effect of instructions on conventional automated perimetry. Invest Ophthalmol Vis Sci. 2000;41(7):2006-13.
9. Bengtsson B, Heijl A. False-negative responses in glaucoma perimetry: indicators of patient performance or test reliability? Invest Ophthalmol Vis Sci. 2000;41(8):2201-4.
10. Katz J, Sommer A, Gaasterland D, Anderson D. Comparison of analytical algorithms for detecting glaucomatous visual field loss. Arch Ophthalmol. 1991;109(12):1684-9.
11. Asman P, Heijl A. Glaucoma Hemifield Test. Automated visual field evaluation. Arch Ophthalmol. 1992;110(6):812-9.
12. Bengtsson B, Heijl A. A visual field index for calculation of glaucoma rate of progression. Am J Ophthalmol. 2008;145(2):343-53.
13. European Glaucoma Society. Terminology and Guidelines for Glaucoma. 3rd ed. Savona: Editrice Dogma S.r.l.; 2008.
14. Chauhan BC, Garway-Heath DF, Goñi FJ, et al. Practical recommendations for measuring rates of visual field change in glaucoma. The British Journal of Ophthalmology. 2008;92(4):569-73.
15. Heijl A, Bengtsson B. The effect of perimetric experience in patients with glaucoma. Arch Ophthalmol. 1996;114(1):19-22.
16. Trick G, Trick L, Kilo C. Visual field defects in patients with insulin-dependent and noninsulin-dependent diabetes. Ophthalmology. 1990;97(4):475-82.
17. Leske M, Heijl A, Hyman L, et al. EMGT Group. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology. 2007;114(11):1965-72.
18. Leske M, Heijl A, Hyman L, Bengtsson B. Early Manifest Glaucoma Trial: design and baseline data. Ophthalmology. 1999;106(11):2144-53.
19. Advanced Glaucoma Intervention Study 2 Visual field test scoring and reliability. Ophthalmology. 1994;101(8):1445-55.
20. Musch D, Lichter P, Guire K, Standardi C. The Collaborative Initial Glaucoma Treatment Study: study design, methods, and baseline characteristics of enrolled patients. Ophthalmology. 1999;106(4):653-62.
21. Rao H, Jonnadula G, Addepalli U, et al. Effect of cataract extraction on Visual Field Index in glaucoma. J Glaucoma. 2013;22(2):164-8.
22. Kerrigan-Baumrind L, Quigley H, Pease M et al. Number of ganglion cells in glaucoma eyes compared with threshold visual field tests in the same persons. Invest Ophthalmol Vis Sci. 2000;41(3):741-8.
23. Reis A, Vidal K, Kreuz A, et al. Nerve fiber layer in glaucomatous hemifield loss: a case-control study with time-and spectral-domain optical coherence tomography. Arq Bras Oftalmol. 2012;75(1):53-8.
24. Jampel H, Vitale S, Ding Y, et al. Test-retest variability in structural and functional parameters of glaucoma damage in the glaucoma imaging longitudinal study. J Glaucoma. 2006;15(2):152-7.
25. Kaushik S, Mulkutkar S, Pandav S, et al. Comparison of event-based analysis of glaucoma progression assessed subjectively on visual fields and retinal nerve fibre layer attenuation measured by optical coherence tomography. Int Ophthalmol. 2014 Dec 13. [Epub ahead of print].

